Gadopiclenol at half the dose of gadobutrol is just as effective for lesion evaluation on contrast-enhanced body MRI exams, according to research published on July 18 in Radiology.
The findings suggest that gadopiclenol shows promise as an alternative to other gadolinium-based contrast agents (GBCAs), which have prompted some concern about the potential for kidney injury, according to a group led by Christiane Kuhl, MD, from Aachen University Hospital in Germany.
"Gadopiclenol offers a substantially higher relaxivity than current state-of-the-art GBCAs," Kuhl and colleagues noted. "Accordingly, gadopiclenol could be used at a dose lower than that approved for other current GBCAs."
GBCAs are generally considered safe, with adverse reactions reported in 0.07% to 2.4% of cases (which tend to be mild). But gadolinium can remain in the body -- especially in those with impaired kidney function -- which can lead to nephrogenic systemic fibrosis. To avoid this complication, researchers have sought to develop contrast agents that don't deposit into body tissues, such as gadopiclenol, a nonionic macrocyclic GBCA.
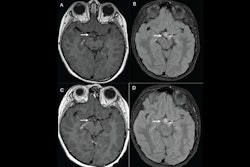
Kuhl's group compared the performance of gadobutrol to gadopiclenol for lesion evaluation via a study that included 260 participants who underwent both gadopiclenol- and gadobutrol-enhanced MRI and had at least one identified lesion. Most patients presented with neoplasms (66%), and the most frequent of these were liver metastases and breast cancer.
The gadopiclenol exam was performed with 0.05 mmol/kg of contrast, while the gadobutrol exam was performed with 0.1 mmol/kg. The exams were read by 18 readers divided into three reading groups to compare the two contrast agents.
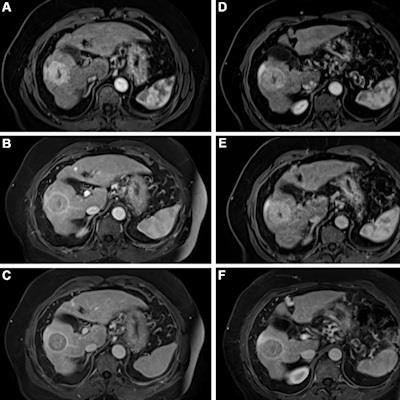
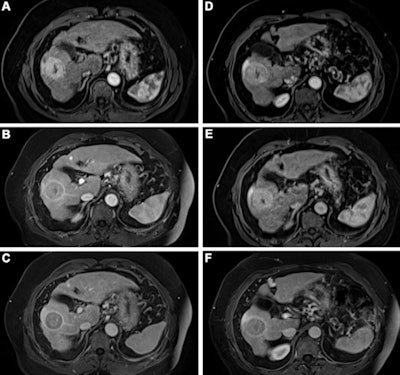 Contrast-enhanced MRI of the liver after administration of gadopiclenol (0.05 mmol/kg) and gadobutrol (0.1 mmol/kg). Axial 3D T1-weighted dynamic contrast-enhanced MRI scans during the (A, D) arterial, (B, E) portal venous, and (C, F) delayed phases in a 66-year-old man with hepatocellular cancer. Images were obtained after administration of gadopiclenol at 0.05 mmol/kg (in A, B, and C) or gadobutrol at 0.1 mmol/kg (in D, E, and F). Images and caption courtesy of the RSNA.
Contrast-enhanced MRI of the liver after administration of gadopiclenol (0.05 mmol/kg) and gadobutrol (0.1 mmol/kg). Axial 3D T1-weighted dynamic contrast-enhanced MRI scans during the (A, D) arterial, (B, E) portal venous, and (C, F) delayed phases in a 66-year-old man with hepatocellular cancer. Images were obtained after administration of gadopiclenol at 0.05 mmol/kg (in A, B, and C) or gadobutrol at 0.1 mmol/kg (in D, E, and F). Images and caption courtesy of the RSNA.The investigators found that gadopiclenol at half the dose of gadobutrol performed comparably for all qualitative visualization parameters and for all readers. The majority of readers across the three groups (ranging from 75% to 83%) reported no preference between the two agents for image quality.
The researchers also reported that the 28 adverse events (12 in patients who received gadopiclenol and 16 in patients who received gadobutrol) were comparable in frequency, intensity, and type. Milder adverse events included injection site pain, nausea, headache, vomiting, and extravasation. There was one acute kidney injury after the use of gadobutrol and one case of renal failure.
The study findings could translate into better patient care, according to the authors.
"[Our] findings suggest that macrocyclic contrast agents with high relaxivity, like gadopiclenol, should preferably be used for contrast-enhanced MRI, in particular for individuals who require multiple contrast-enhanced MRI examinations and for susceptible individuals, such as pediatric patients and patients with impaired renal function," they concluded.
Clinicians must continue to discern which agent is best for their patients, taking cost into consideration, wrote Mustafa Bashir, MD, of Duke University in Durham, NC, and colleague Kerry Thomas, MD, of the University of North Carolina at Chapel Hill, in an accompanying commentary.
"As with all medical decision-making, the potential benefits of any new pharmaceutical agent, including GBCAs, must be weighed against costs," they noted.
The complete study can be found here.