Post-treatment MRI can accurately predict treatment response for early triple-negative breast cancer, according to research published July 1 in Radiology.
A team led by Toulsie Ramtohul, MD, from the Curie Institute in Paris, France, found that their logistic regression model, which used radiologic complete response, nodal involvement, and Ki-67 indexing, achieved high predictive value for complete pathologic response in early triple-negative breast cancer following neoadjuvant chemoimmunotherapy.
“Patients with radiologic complete response, node-negative disease, and high Ki-67 may be suitable candidates for trials evaluating surgical omission,” the Ramtohul team wrote.
MRI is the most accurate imaging modality for analyzing tumor response and residual disease after neoadjuvant chemoimmunotherapy. It can provide assessment data of changes in lesion size, volume, and enhancement patterns.
Ramtohul and colleagues evaluated the performance of post-treatment MRI in predicting tumor response in women with triple-negative breast cancer who were treated with neoadjuvant chemoimmunotherapy.
The multicenter prospective study, which took place between 2021 and 2024, included 259 women. For the study, the team developed and validated a multivariable logistic regression model incorporating radiologic complete response, nodal involvement, and Ki-67 index. It also tested a radiomic score using shape and first-order features in cases with residual enhancement, meaning these cases did not achieve radiologic complete response.
The researchers included 175 of the women in a training set and 84 women in an external test set.
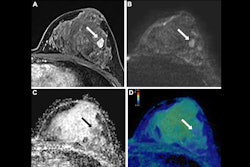
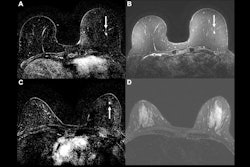
 Example of a true-positive radiologic complete response at dynamic contrast-enhanced MRI in a 53-year-old woman who underwent neoadjuvant chemoimmunotherapy for triple-negative breast cancer. (A-C) Axial T1-enhanced dynamic contrast-enhanced MRI scans acquired before treatment. The cancer appeared as a spherical 20-mm mass enhancement in the left breast, indicated by arrows on (A) native and (B) subtracted images. (C) Native image shows the absence of axillary node involvement (arrowhead). (D, E) At post-treatment MRI, no residual enhancement was observed within the initial tumor bed, marked by a coil (dashed arrow), on (D) native and (E) subtraction scans. (F) Pathologic examination after chemoimmunotherapy revealed no residual invasive cancer in the breast or lymph nodes, confirming a pathologic complete response (hematoxylin-eosin stain; magnification, 200×).RSNA
Example of a true-positive radiologic complete response at dynamic contrast-enhanced MRI in a 53-year-old woman who underwent neoadjuvant chemoimmunotherapy for triple-negative breast cancer. (A-C) Axial T1-enhanced dynamic contrast-enhanced MRI scans acquired before treatment. The cancer appeared as a spherical 20-mm mass enhancement in the left breast, indicated by arrows on (A) native and (B) subtracted images. (C) Native image shows the absence of axillary node involvement (arrowhead). (D, E) At post-treatment MRI, no residual enhancement was observed within the initial tumor bed, marked by a coil (dashed arrow), on (D) native and (E) subtraction scans. (F) Pathologic examination after chemoimmunotherapy revealed no residual invasive cancer in the breast or lymph nodes, confirming a pathologic complete response (hematoxylin-eosin stain; magnification, 200×).RSNA
Radiologic complete response at MRI showed predictive value for complete pathologic response with an area under the receiver operating characteristic curve (AUC) of 0.83. The combined model, meanwhile, achieved an AUC of 0.88 in the test set.
The team also measured the radiologic complete response false-discovery rate in its study. This is the proportion of radiologic complete response cases that were actually nonpathologic complete response or residual disease missed at breast MRI. In node-negative patients with Ki-67 greater than 30%, this false-discovery rate was 3.6% in the training set and 3.5% in the test set. The researchers also reported that cancers were limited to residual cancer burden I.
Finally, the team’s model that included the radiomics score and lesion count achieved an AUC of 0.80 in cases of nonradiologic complete response.
Despite the success described in their study, the authors called for longer-term data to assess the risk of distant recurrence.
“A more comprehensive radiomic approach incorporating texture and higher-order features could be explored in future studies with larger datasets,” they added.
The results could serve as a reliable guide for clinical management of triple-negative breast cancer, according to an accompanying editorial written by Natsuko Onishi, MD, PhD, from the University of California, San Francisco.
She also suggested that incorporating more imaging data, including multiple time points before, during, and after neoadjuvant chemoimmunotherapy, could improve understanding of the disease under this new treatment.
“This approach may lead to the identification of imaging biomarkers relevant to chemoimmunotherapy, thereby contributing to the development of more personalized and effective treatment strategies,” Onishi wrote. “Continued research in this area will be essential to optimize patient outcomes and advance the field of breast cancer care.”
The full study can be accessed here.