An apparent diffusion coefficient (ADC) cutoff based on MRI with diffusion-weighted imaging (DWI) could detect more breast lesions, suggest findings published June 10 in Radiology.
Applying this ADC cutoff improved the performance of preoperative MRI in diagnosing additional enhanced lesions in the ipsilateral and contralateral breasts in breast cancer patients, wrote a team led by Su Min Ha, MD, PhD, from Seoul National University Hospital in South Korea.
“The application of the ADC cutoff value resulted in an improved diagnostic yield,” Ha and colleagues wrote. “Thus, our findings may help reduce patient anxiety and healthcare costs associated with unnecessary preoperative procedures and surgeries.”
Although dynamic contrast-enhanced (DCE) MRI has high sensitivity, it is not recommended for preoperative imaging since it does not improve outcomes. Furthermore, radiologists are challenged with overlapping MRI features between benign and malignant lesions.
However, the researchers noted the potential of multiparametric MRI, which combines DCE-MRI with DWI, in improving diagnostic accuracy. This is because of DWI’s ability to assess lesion microstructure and cellularity. Previous studies also suggest that the ADC metric can be taken from DWI findings to measure in vivo diffusion.
Ha and co-authors studied the potential of DWI with an ADC cutoff in evaluating additional lesions found on preoperative MRI in breast cancer patients.
They reviewed MRI data collected between 2019 and 2021 from 219 women with an average age of 50.8 years. This data included a development group of 292 additional lesions categorized as BI-RADS 3 or higher. Two breast radiologists independently evaluated additional enhanced lesions and measured the ADC values. The research team also included 104 women with 133 additional lesions in a validation group.
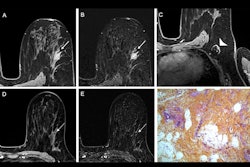
 Images show ADC measurements for a BI-RADS 4 lesion in the left contralateral breast of a 49-year-old woman who underwent preoperative MRI for right invasive ductal carcinoma. (A) Axial dynamic contrast-enhanced MRI scan reveals a 1-cm oval, circumscribed, homogeneous mass (arrow) in the left breast. (B-D) At axial diffusion-weighted imaging (DWI; b value = 1,200 sec/mm2), the lesion (arrow) has high signal intensity (B), with an ADC of 1.2 × 10−3 mm2/sec, and is shown on the ADC map calculated using b values of 0 and 800 sec/mm2 (C) and fused DWI and ADC image (D). Biopsy revealed a benign fibroadenoma.RSNA
Images show ADC measurements for a BI-RADS 4 lesion in the left contralateral breast of a 49-year-old woman who underwent preoperative MRI for right invasive ductal carcinoma. (A) Axial dynamic contrast-enhanced MRI scan reveals a 1-cm oval, circumscribed, homogeneous mass (arrow) in the left breast. (B-D) At axial diffusion-weighted imaging (DWI; b value = 1,200 sec/mm2), the lesion (arrow) has high signal intensity (B), with an ADC of 1.2 × 10−3 mm2/sec, and is shown on the ADC map calculated using b values of 0 and 800 sec/mm2 (C) and fused DWI and ADC image (D). Biopsy revealed a benign fibroadenoma.RSNA
The researchers highlighted the best ADC cutoff being 1×10−3 mm2/sec. Compared with DCE-MRI alone, this cutoff value derived from DWI increased specificity from 28.7% to 73.1% (p < 0.001). However, it also decreased sensitivity from 99.2% to 89.6% (p < 0.001).
In the validation group, 48 lesions (36.1%) were diagnosed as cancer. The team noted trends when applying the ADC cutoff taken from the development group for ipsilateral and contralateral lesions. This included specificity increasing with the ADC cutoff included, but with the caveat of decreased sensitivity.
Comparison between detected additional lesions on multiparametric MRI with ADC cutoff | |||
Measure | DCE-MRI alone | With DWI-derived ADC cutoff value | p-value |
Overall |
|
|
|
Sensitivity | 97.9% | 83.3% | 0.01 |
Specificity | 21.2% | 68.2% | < 0.001 |
Ipsilateral lesions |
|
|
|
Sensitivity | 97.1% | 85.3% | 0.052 |
Specificity | 8.7% | 65.2% | < 0.001 |
Contralateral lesions |
|
|
|
Sensitivity | 100% | 78.6% | < 0.001 |
Specificity | 35.9% | 71.8% | < 0.001 |
Despite the results, the study authors cautioned that radiologists should be aware of the potential of missing some malignant lesions. They also noted that 4.1% (12 of 292) of cancers in the development group were missed, and 5.2% (seven of 133) were missed in the validation cohort.
The authors wrote that the ADC threshold should not be the sole parameter involved in characterizing breast lesions.
“Additional joint consideration of the morphologic and kinetic features of DCE-MRI could help avoid false-negative findings,” they wrote.
Still, advancing DWI to its current stage of clinical validation is a notable achievement in breast imaging technology, according to an accompanying editorial written by Maya Honda, MD, PhD, from Kyoto University Hospital, and Mami Iima, MD, PhD, from Nagoya University, both in Japan.
The two wrote that achieving the best balance between sensitivity and specificity will require multicenter trials “to establish consensus protocols that maximize patient benefits through reduced reoperations, fewer unplanned mastectomies, and decreased health care costs across diverse patient populations and clinical settings.”
The full study can be read here.