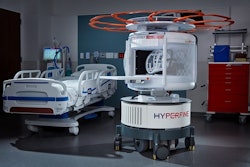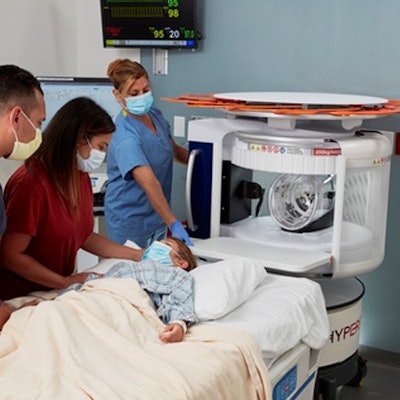
Hyperfine is touting that its study, Portable MRI for Children with Neurological Injury -- A Pilot Study using Hydrocephalus as an Index Condition (HOPE PMR), has complete enrollment.
The multicenter study aims to provide more accessible care for pediatric hydrocephalus patients and validate the company's Swoop system, a portable MR brain imaging system cleared by the U.S. Food and Drug Administration (FDA). The study is using pediatric hydrocephalus as an index condition to investigate whether Swoop system images can be used to accurately detect shunt malfunctions and assess ventricular size.