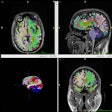
AIRAmed has received U.S. Food and Drug Administration (FDA) clearance to market its AIRAscore AI software in the U.S. for assessing brain volumes on MRI scans.
The German medical technology company's software received 510 (k) clearance on September 19 from the FDA. AIRAscore is a deep learning algorithm provides quantitative assessment of brain volume on MRI scans in as little as five minutes and may be helpful in assisting physicians in early detection of Alzheimer’s and other dementias, the company said.
Specifically, AIRAscore offers auto segmentation of grey matter, white matter, cerebrospinal fluid, and T1-weighted hypointensities and provides detailed measurements of all brain lobes, midbrain and pons, hippocampus, cerebellum, and ventricular systems, AIRAmed noted.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)




.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)