Pune, India-based InMed.AI (InMed Prognostics) has received 510(k) clearance from the U.S. Food and Drug Administration for its NeuroShield technology for use in MRI.
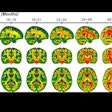
NeuroShield is a fully automated brain geometry- and cloud-based platform designed for neurologists and neuroradiologists. The software takes 3D MR images as inputs and automatically calculates brain volumes that can assist physicians in developing treatment plans for patients with neurodegenerative diseases such as dementia, Alzheimer's disease, Parkinson's disease, and epilepsy, InMed said.
In a trial with 280 patients drawn from different parts of the U.S., NeuroShield demonstrated high accuracy across scanners of different magnetic strengths, clinical subgroups, gender, age, and slice thickness, according to the company. NeuroShield is currently in clinical use in more than 220 sites across the world, InMed added.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)

.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)