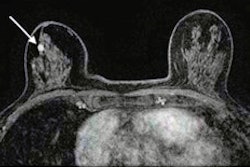
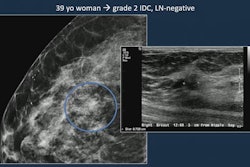
Several neoadjuvant chemotherapy response characteristics are often left out of breast MRI reports, suggest findings presented May 7 at the American Roentgen Ray Society (ARRS) annual meeting.
In a talk delivered at the conference, Sheida Ebrahimi, MD, from the University of California, San Diego presented her team’s study, which showed that little to no reports indicate the timing of chemotherapy, changes in background parenchymal enhancement (BPE), and the type of response in the findings section.
“This single-institution, quality improvement project suggests that there is an opportunity to improve the reporting of responses to neoadjuvant chemotherapy,” Ebrahimi said.
Contrast-enhanced breast MRI has high sensitivity and can evaluate response to neoadjuvant chemotherapy for breast cancer patients. However, the researchers noted inconsistent breast MRI reporting of chemotherapy.
Ebrahimi and colleagues assessed breast MRI reporting for chemotherapy response with the goal of identifying areas for improvement and understanding satisfaction among referring clinicians regarding current reports. This includes gathering their insights on reporting adequacy.
 Research presented at the ARRS annual meeting highlighted the need for more detailed breast MRI reporting for neoadjuvant chemotherapy responses. One finding highlighted was that BPE changes are underreported.ARRS
Research presented at the ARRS annual meeting highlighted the need for more detailed breast MRI reporting for neoadjuvant chemotherapy responses. One finding highlighted was that BPE changes are underreported.ARRS
The retrospective study included 30 breast MRI reports that documented neoadjuvant chemotherapy response. Five radiologists with between one and 30 years of experience generated the reports. The researchers analyzed the reports for key elements and details, including the following: indication, BPE, fibroglandular tissue, the timing of chemotherapy, type of malignancy, change in BPE, the pattern of response, the extent of disease, type of response, and changes in tumor size.
Finally, the team issued a survey to referring clinicians with questions about satisfaction with the reports and whether they found them suitable for tumor assessment. The referring clinicians included surgeons, oncologists, radiation oncologists, and advanced care practitioners.
All reports included the indication, BPE, and fibroglandular tissue. However, just 9% indicated the timing of chemotherapy, none mentioned BPE changes, and 4% mentioned the type of response in the findings section.
The team also reported the following:
- 77% of reports noted the type of treatment response in the impression.
- 53% included preoperative MRI evaluation.
- 25% reported the extent of the disease.
- 83% included changes in tumor size.
- 71% noted T2 signal intensity changes.
- 4% mentioned the change in kinetics in the target lesion.
Additionally, no report in the impression included the pattern of response, 95% included the maximum extent of disease, and 40% included baseline size.
Survey results showed that the referring clinicians had a moderate satisfaction level with the reports, with 67% being satisfied and 33% very satisfied. Also, 75% agreed that current reports were adequate for tumor assessment, with 8% strongly agreeing and 17% remaining neutral. Finally, 50% of clinicians wanted the response pattern to be included in reports, and 100% stressed the importance of including the extent of disease in reporting.
Ebrahimi suggested that improving collaboration between radiologists and referring clinicians makes way for more accurate and comprehensive breast MRI reporting, which can lead to improved patient care. She added that as part of this project, a structured report will be instituted, and the team will reassess the report content and clinician satisfaction with reporting.
“I’m hoping that next year, we can come back and say that we’ve improved in our reporting,” Ebrahimi said.