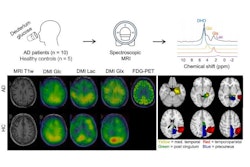
Deuterium metabolic imaging (DMI) can reveal impaired brain glucose metabolism in patients with Alzheimer’s disease, according to a study published July 16 in Radiology.
In a pilot study, a group at University of Cambridge in Cambridge, England, found that the technique was feasible using a 3-tesla MRI system, but more research will be required to support its use as a diagnostic tool.
“Deuterium metabolic imaging (3 tesla) demonstrated a reduction in oxidative glucose metabolism at whole-brain spectroscopy, but no evidence of regional signal changes, in participants with Alzheimer’s disease,” noted lead author Alixander Khan, a doctoral student at the university.
PET imaging studies using F-18 FDG radiotracer show that patients with Alzheimer’s disease have reduced glucose metabolism in the posterior cingulate and temporoparietal regions of the brain. However, the use of PET remains limited by its higher cost and lower availability than MRI, according to the authors.
DMI is an emerging method for imaging tissue metabolism based on MR spectroscopic detection of deuterium-labeled compounds, they explained. In initial DMI studies in humans, magnetic fields of 4-tesla and 9.4-tesla have shown promise, yet performing DMI with a 3-tesla MRI system could facilitate its use in clinical settings, as these scanners are more widely available, they noted.
Khan and colleagues recruited 10 participants with Alzheimer's disease (mean age, 68 years old, eight males) and nine cognitively healthy control participants. Participants received an oral dose of deuterium-labeled glucose (0.75 g/kg) and then underwent DMI with a 3-tesla system that employed a custom birdcage head coil.
For the analysis, the researchers calculated ratios of 2H-glucose, 2H-glutamate, 2H-glutamine (2H-Glx), and 2H-lactate spectroscopic peak signals to 2H-water peak signal among the participants.
According to the findings, the whole-brain images demonstrated a reduced ratio of 2H-Glx to 2H-glucose peak signals in participants with Alzheimer’s disease compared with control participants (0.41 vs. 0.58; p = 0.04), which suggests an impairment of oxidative glucose metabolism in Alzheimer’s.
“However, there was no evidence of localization of these changes to the expected regions of metabolic impairment at [magnetic resonance spectroscopic imaging], presumably due to insufficient spatial resolution,” the investigators wrote.
Ultimately, this was an initial study exploring the role of DMI in Alzheimer’s disease patients and adds to the body of evidence on the usability of DMI in humans, the authors noted.
“Further investigations are warranted to determine whether DMI could provide regional information following technical coil and scan protocol refinements sufficient to provide the necessary regional signal-to-noise ratio at 3T, or whether greater magnetic field strength would be required,” the group concluded.
Access to the full study is available here.