Lung MRI developer Polarean Imaging has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its MRI chest coil to be used with GE HealthCare's (GEHC) 3-tesla (3T) MRI scanners to visualize xenon-129 nuclei.
Polarean now supports xenon MRI scanning of both clinical and research patients on GEHC, Philips, and Siemens Healthineers scanners.
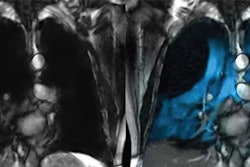
Polarean’s GEHC-compatible Xenoview 3T chest coil is a flexible, single-channel, transmit-receive radiofrequency coil for imaging xenon-129 nuclei. This is done while a patient is positioned inside a GEHC Signa Premier 3T or Discovery MR750 3T MRI scanner equipped with the latter's multinuclear spectroscopy capability.
The coil is to be used in conjunction with hyperpolarized xenon-129 for oral inhalation for the evaluation of lung ventilation in adults and pediatric patients, aged 12 years and older. Polarean said the coil supports institutions with GEHC-compatible MRI systems looking to adopt xenon MRI.