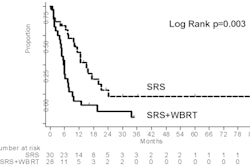
Brachytherapy device developer Xoft of Sunnyvale, CA, has received clearance from the U.S. Food and Drug Administration (FDA) for a skin and surface treatment applicator for use with its Axxent electronic brachytherapy system to deliver surface brachytherapy, including intraoperative radiation therapy.
The FDA clearance allows the applicator to be used on any external or internal surface of the body where radiation therapy is indicated.
The electronic brachytherapy treatment platform is designed to deliver localized, nonradioactive, isotope-free radiation treatment in minimally shielded clinical settings under the supervision of a radiation oncologist.
Related Reading
Xoft's Axxent used in IORT procedure, October 1, 2008
Rhode Island Hospital uses Axxent for endometrial cancer, September 29, 2008
Xoft debuts mobile brachytherapy vehicles, September 18, 2008
Xoft launches electronic brachytherapy registry, June 26, 2008
Xoft taps new CMO, June 18, 2008
Copyright © 2009 AuntMinnie.com