An advisory committee to the U.S. Centers for Medicare and Medicaid Services (CMS) tried to get a handle on the thorny issue of radiation therapy for prostate cancer last week. But the panel encountered a thicket of literature that despite its volume failed to offer much guidance as to whether CMS should increase Medicare reimbursement.
It's not that recent data have not been published about the effectiveness of radiation therapy treatments for prostate cancer. Almost 1,300 peer-review articles have been published on this subject between 2007 and 2009. But, collectively, they didn't provide the answers that a Medicare advisory committee wanted to know: how effective and beneficial specific radiation therapy treatments have been for localized prostate cancer, and the frequency and severity of morbidities associated with them.
Medical professionals and prostate cancer experts assembled at a Medicare Evidence Development and Coverage Advisory Committee (MedCAC) meeting in Washington, DC, on April 21 to discuss the effects of radiotherapy for the treatment of localized prostate cancer. The meeting reconfirmed that treating prostate cancer, with or without radiation therapy, is an inexact science, many treatment choices exist, and patients present with a variety of factors that are not easy to categorize, measure, or compare.
More than 2 million men in the U.S. have been diagnosed and are living with prostate cancer. One in six men will develop prostate cancer during his lifetime. In 2009, an estimated 192,000 men were diagnosed with the disease at a median age of 67, according to the American Cancer Society (ACS). As the baby boomer generation ages, this number is expected to skyrocket, especially because widespread prostate-specific antigen (PSA) testing has doubled detection rates, according to government reports.
Although prostate cancer is responsible for 10% of deaths in men and an estimated 27,300 died from the disease in 2009, for the vast majority, low-grade localized prostate cancer grows slowly. Death from other causes, including natural death from old age, may occur before well before treatment is needed.
Are low-risk prostate cancer patients with localized disease receiving costly treatments they don't really need -- treatments that do not benefit them and that may cause significant, debilitating morbidities? Would monitoring be just as effective? Is radiation therapy treatment excessive?
The MedCAC committee sought to find out.
Appropriateness questions
The elephant in the room at the hearings was the appropriateness of paying for radiation therapy, and the looming issue of whether CMS can afford to do so. With approximately 18 million male enrollees in Medicare, a number that's rising daily, the cost could be staggering.
The 17-member MedCAC panel heard presentations during the day-long meeting from 11 invited speakers and an additional 15 radiation oncologists, representatives of nonprofit organizations, and prostate cancer patients. Members of the panel included a CMS director and a mix of radiologists, radiation oncologists, surgeons, urologists, medical physicists, molecular oncologists, health insurance representatives, and health policy, economics, and health assessment experts.
The panel's assignment was to determine if enough evidence exists to prove whether radiation therapy as a whole -- and the various types of radiation therapy, individually -- improves health outcomes for patients with localized prostate cancer compared to clinical monitoring. The panel also was asked to determine what type of additional evidence on the impact of radiotherapy is needed to improve decision-making when treating the disease, as well as actions that the medical academic and treatment community should take to address evidentiary gaps in information.
What did the committee learn?
AHRQ tech assessment
A technology assessment report prepared by the Tufts Medical Center Evidence-based Practice Center (EPC) of Boston for the Agency for Healthcare Research and Quality (AHRQ) in Rockville, MD, took center stage. The report, published March 25, 2010, provided a meta-analysis and comparative evaluation of peer-review literature on randomized controlled trials and nonrandomized direct comparative studies of men with clinically localized prostate cancer that reported clinical outcomes for T1 or T2 disease. The report covered 36 months, starting January 2007, and updated a prior AHRQ report published in 2008.
The Tufts research team, headed by associate director Dr. Stanley Ip, identified 1,283 articles, of which 53 met its inclusion criteria. The group added nine studies referenced in the prior report, for a total of 62 studies to analyze, rank for content and relevancy, and compare.
The objectives of the meta-analysis were:
- To determine the benefits and harms of radiotherapy provided for clinically localized prostate cancer compared to watchful waiting, clinical observation, or active surveillance.
- To identify and compare the benefits and harms of different forms of radiotherapy. The treatments included all types of external-beam radiation therapy (EBRT), stereotactic body radiation therapy (SBRT), and brachytherapy, as well as combination treatments of these.
- To identify the specific patient characteristics that affect outcomes of different forms of radiotherapy. Outcomes of interest included overall survival, prostate cancer-specific survival, biochemical (PSA)/metastatic and/or clinical progression-free survival, quality of life, and health status. Adverse events to be evaluated included bowel, bladder, and sexual dysfunction.
Simply stated, the researchers were tasked with comparing apples to oranges, grapefruits, pomelos, and tangerines. The heterogeneity of the clinical trials made analysis impossible. So the research team reported that there was lack of evidence on all counts to answer the questions.
Specifically:
- No comparisons could be made between patients who received external-beam radiation therapy and patients who either received no treatment or for whom initial treatment was postponed.
- African-American men, in particular, and men of other minorities were underrepresented.
- There were no studies comparing EBRT and high-dose-rate brachytherapy. Nor were there studies comparing SBRT with any other radiation modality.
- A number of comparative studies showed inconsistent results.
- Few studies were conducted on the potential effects of different patient characteristics on treatment outcomes.
- No studies were found related to the frequency of errors made that could cause toxicities.
Data did suggest that higher EBRT dose is associated with increased rates of long-term biochemical control compared with lower dose, and that brachytherapy is associated with more genitourinary toxicity and less gastrointestinal toxicity compared with EBRT, according to the Tufts research team.
The draft AHRQ report may be read in its entirety through May 7, 2010, by clicking here.
AUA and ASTRO Comments
Dr. Carl Olsson, chief medical officer of Integrated Medical Professionals of Melville, NY, spoke on behalf of the American Urological Association (AUA) headquartered in Linthicum, MD.
"Newly diagnosed prostate cancer patients have over 20 unique management choices," Olsson said. "Selecting the right choice is a complex process dependent upon age, state of health, life expectancy, tolerance for risk, and concern about side effects."
Olsson pointed out that a recent review of 85,000 men diagnosed with prostate cancer determined that treatment was often influenced by the type of physician managing the patient. Generalizing, he pointed out that the majority of patients who only consulted with a urologist would elect surgery, but if they also saw a radiation oncologist, 78% of this group would elect radiation therapy.
If men consulted with a urologist and an oncologist, half of this group would elect surgery and the rest would equally choose radiation therapy, watchful waiting, or androgen deprivation therapy.
Olsson summarized the AUA's independent analysis of peer-review literature to determine if it could answer the questions posed to the MedCAC panel. For a disease with an incidence as high as prostate cancer, there is an alarming paucity of category 1 evidence-based treatment results, Olsson noted.
The AUA could not identify studies comparing radiation therapy with no treatment. All reports of active surveillance suffer from small size, not exceeding 500 patients, and relatively short follow-up. Olsson stressed that any promise that the patient will be curable when a trigger point is reached is fallacious, as this has not been well studied.
He pointed out that the National Comprehensive Cancer Network recommends active surveillance for both very low-risk and low-risk cancer patients with less than 20 and 10 years likelihood of survival, respectively, an estimate of 40% of all newly diagnosed patients.
New tests are needed to identify prostate cancer aggressiveness and determine which treatments should be provided. The AUA reviewed two tests that differentiate the killer cancers from those that are survivable: Trichomonas vaginalis seropositivity and PTEN loss with ERG/ETV1 gene rearrangement. Noting that the authors of these studies, which were designed to predict long-term failure of treatment, suggested that such tests could be helpful in stratifying patients at the time of diagnosis, the AUA strongly endorses expanding these studies and similar research to help decision-making.
In its testimony, the American Society for Radiation Oncology (ASTRO) of Fairfax, VA, supported the use of radiation therapy, providing data on how EBRT and brachytherapy are treating prostate cancer without significant side effects. ASTRO stated that SBRT shows promise as a rapid, noninvasive treatment but cautioned that the technology needs further study in clinical trials to fully capture and document quality-of-life measures. Detailed remarks may be read by clicking here.
Dr. Anthony Zeitman, president of ASTRO and a radiation oncologist specializing in genitourinary cancers at Massachusetts General Hospital in Boston, spoke informally.
"ASTRO understands the issue of overtreatment," he said. "But prostate cancer is a spectrum of diseases from mild to fatal, and there is a huge gray area in between. There is no lack of evidence about radiation therapy treatment. There is just a lack of comparative evidence. That is the deficit."
Appealing for no hasty decisions by MedCAC or CMS, Zeitman noted that there is plenty of evidence of efficacy and low morbidity, just not much comparative evidence. He referenced ongoing randomized trials, whose data will become available within this decade. This included the "mother of all randomized trials," the Prostate Testing for Cancer and Treatment (ProtecT) study in the U.K., which is evaluating the outcomes of surgery, radiation therapy, and active monitoring of more than 94,000 men.
RTOG testimony
Dr. Howard Sandler, chair of radiation oncology at Cedars-Sinai Medical Center in Los Angeles, and chair of the Radiation Therapy Oncology Group (RTOG) Genitourinary Cancer Committee, emphasized that no single treatment option for patients with localized prostate cancer is appropriate for every patient, and no single treatment option is clearly superior.
Sandler pointed out that the terms "watchful waiting" and "active surveillance" should not be used interchangeably. Watchful waiting, in which physicians carefully monitor the patient and provide palliative treatment if symptoms or metastases develop, is no longer practiced, according to Sandler. Rather, patients undergo active surveillance, in which early-stage, low-grade prostate cancer is regularly monitored, and physicians perform PSA testing and periodic biopsies. If cancer progression is observed, patients will receive more radical treatment.
However, active surveillance has its risks and is still under conceptual development. It has not been studied long enough or on a broad enough scale to accurately quantify mortality or morbidity. Uniformity is lacking within the medical profession about what active surveillance actually means, what patients are appropriate to elect active surveillance, and even what qualifies as disease progression.
"Active-surveillance clinical trials experience inconsistent tolerance and compliance by patients. Patients may suffer fear and anxiety, and there is a risk of progression," Sandler said.
Sandler referenced a Scandinavian study of two trials in which receiving both radiation therapy and endocrine treatment reduced local recurrence and mortality compared to endocrine treatment alone (Lancet, January 24, 2009, Vol. 373:9660, pp. 301-308), noting that radiation therapy made a statistically significant difference. He also referenced clinical trials showing that dose escalation leads to improved local and biochemical control.
New for fiscal 2010 are U.S. Department of Defense Prostate Cancer Research Program (PCRP) Impact Awards, whose funds can be applied to studies distinguishing lethal from indolent prostate cancer. Applicants are expected to address focus areas in biomarkers, genetics, tumor biology, survivorship, therapy, and imaging. Sandler expressed hope that funds for new clinical trials might help with the answers MedCAC is seeking.
Dr. Peter Grimm of the Prostate Cancer Treatment Center in Seattle represented the Prostate Cancer Results Study Group, a 25-member group of prostate cancer treatment physicians working in the U.S., Europe, and Australia. This group identified 603 articles published between 2000 and 2009 related to treatment and presented its research findings. The group noted that most studies fail to risk-stratify or follow their patient cohort for more than five years, and no good studies have been published that compare survival rates among the various treatment options.
Brachytherapy and CyberKnife proponents
Brachytherapy advocates who made presentations included Dr. Gregory Merrick, a radiation oncologist at Schiffler Cancer Center of Wheeling, WV, and Dr. Jonathan Briers, vice president of medical affairs of Nucletron in Veenendaal, the Netherlands, who submitted a written presentation only, as volcanic ash canceled his flight to Washington.
Both made the argument, using published clinical trial results, that when administered appropriately, brachytherapy was safe; as effective as other radiation therapy treatments with respect to outcomes; took only a few days and, therefore, represented a more viable treatment option for many transportation-constrained or working patients; and had the lowest cost to administer.
Three scheduled speakers and 12 unscheduled speakers, many representing user physicians from the nonprofit CyberKnife Coalition as well as patients, argued for increased support of CyberKnife treatment. This SBRT treatment currently is not compensated by Medicare in 13 states, a subject of significant contention.
Dr. Sean Collins, Ph.D., director of the CyberKnife program at Georgetown University Hospital's Lombardi Comprehensive Cancer Center in Washington, DC, identified articles reporting five clinical trials, representing a total of 631 patients with localized prostate cancer, that had been published since ASTRO published its report on SBRT use in September 2008. He provided comparisons with other radiation therapy treatments, noting that SBRT ranked highest, along with high-dose-rate brachytherapy, at preserving sexual function for 81% of patients, compared with 60% for EBRT.
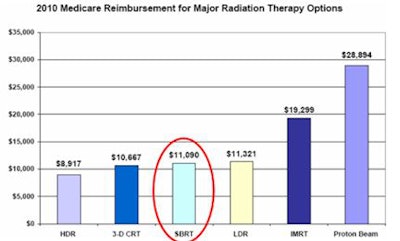
Collins also caught the attention of the panel with a graph showing 2010 Medicare reimbursement for six types of radiation therapy. These ranged from a low of about $8,900 for high-dose-rate brachytherapy to more than $19,000 for intensity-modulated radiation therapy and a high of almost $29,000 for proton-beam therapy. SBRT fell squarely in the middle at $11,090.
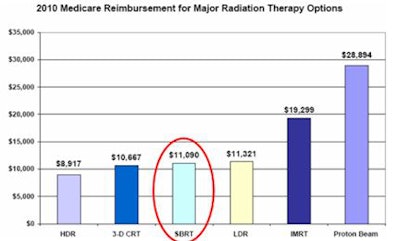 |
| Image courtesy of the Advisory Board Company. |
Chrissie Kotwica, a registered nurse and data manager/physician liaison at CyberKnife Centers of Miami and Palm Beach, represented the CyberKnife Coalition, treatment centers that have now performed more than 80,000 CyberKnife procedures worldwide. She presented unpublished data of 250 prostate cancer patients who responded to a still-open 2010 voluntary survey. Respondents overwhelmingly supported their treatment outcomes.
Unscheduled speakers at the MedCAC hearing were more vocal in their support of this SBRT treatment, particularly regarding short treatment times and lack of lasting or late debilitating side effects such as incontinence and sexual function. Most patients said that their doctors did not mention this treatment, and they learned of it through friends and from Internet Web sites. Some said that if they had not undergone this treatment, they would have opted for active surveillance.
About active surveillance
Dr. Andrew K. Lee, associate professor of radiation oncology at M. D. Anderson Cancer Center at the University of Texas in Houston, focused his presentation on the proven benefits of radiation therapy and the uncertainties associated with active surveillance.
"Active surveillance does require monitoring, and repeat biopsies are not without their own side effects. Many patients on active surveillance get hormone therapy for the rest of their lives, and chemical castration for these men should not be considered as conservative therapy," he stressed.
Lee cautioned the panel to avoid lumping all T1 and T2 localized prostate cancer together, because it can be stratified by low, intermediate, and high risk. "The low-risk patients can probably be addressed with monotherapy, but if they progress to intermediate and high risk, they are going to need additional therapies. Untreated cancers have a tendency to progress. In order to get the same cure fraction, their subsequent treatment can be potentially more toxic as well as costing more money. As they age, these patients may not be up to it."
Lee made a case for using higher doses of radiation therapy. They provide a clinical benefit, he said, and specialized techniques using newer technology are needed to deliver high doses safely with low morbidity. As did other speakers, Lee noted during the meeting that evolving technologies make treatments a moving target when trying to standardize comparisons.
Conclusion
Clifford Goodman, Ph.D., senior vice president of the Lewin Group, a healthcare consulting firm in Falls Church, VA, chaired the MedCAC committee. Having kept a sense of humor and pragmatism while seeking solutions to a global phenomenon that needs strong evidence about the most effective ways to treat localized prostate cancer, he summarized the findings:
"Is there good evidence of head-to-head comparisons? No," he said. "Is there good evidence on patient outcomes rather than biomarkers? No. Is there evidence covering clinically important time frames? Not enough. Is there an apparent commitment to generate the comparative effectiveness research we need? We haven't seen much of it to date, but there have been suggestions as to what needs to be done."
Dr. Marcel Salive, director of the coverage and analysis group in CMS' medical and surgical services division, stated that it was difficult to draw conclusions from the evidence, and this would create difficulties for Medicare to make a national coverage decision in this area.
"Many treatment and effectiveness questions need to be answered," he concluded. "Hopefully Medicare can push in that direction to get some of the needed studies going."
By Cynthia E. Keen
AuntMinnie.com staff writer
April 26, 2010
Related Reading
Radiation for prostate cancer lacks data: U.S. panel, April 22, 2010
U.S. Medicare panel to weigh prostate treatments, April 19, 2010
Higher RT dose improves outcomes for prostate cancer patients, December 14, 2009
SBRT for early prostate cancer shows promising results, April 9, 2009
NY Times: CyberKnife Medicare coverage varies in U.S., December 23, 2008
Copyright © 2010 AuntMinnie.com



















