For the past decade, breast brachytherapy has been used as a treatment for low-risk patients following breast conservation surgery. Although its long-term effectiveness is still unknown, its use has been climbing steadily, according to an article in the January 10 issue of the Journal of Clinical Oncology.
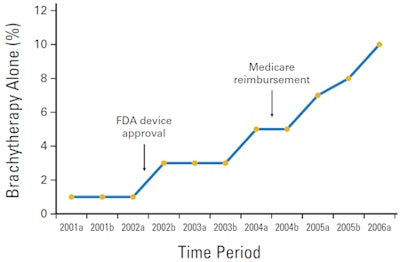
Researchers from MD Anderson Cancer Center at the University of Texas in Houston found that over a six-year time period, from 2001 through June 2006, breast brachytherapy as the sole radiation therapy modality in women ages 65 and older increased from 1% to 10%. However, despite the growth, there have been no long-term clinical studies as to the technology's effectiveness (J Clin Oncol, January 10, 2011, Vol. 29:2, pp.157-165).
Medicare database
To track brachytherapy's rise, the researchers examined procedure growth within the MarketScan Medicare Supplemental database, a large, nationwide employment-based claims database that includes Medicare beneficiaries with private supplemental insurance.
The study did not include the use of same-day-as-surgery electronic brachytherapy, in which a single radiation therapy treatment is administered in the operating suite immediately following breast conservation surgery.
The researchers identified 6,882 women diagnosed with invasive breast cancer at age 65 or older, who had undergone breast conservation surgery followed by some form of radiation therapy treatment between January 2001 and July 2006. Ninety-five percent of these women had conventional electron-beam radiation therapy. The remaining 5%, a total of 333 women, had breast brachytherapy exclusively.
For this group of 333, this equated to four patients in 2001, 17 in 2002, 47 in 2003, 89 in 2004, 112 in 2005, and 64 in the first half of 2006. In addition to patients with low-risk, localized breast cancer with no nodal involvement, the group included several patients with metastatic disease (2%) and several patients with axillary nodal involvement (3%).
This rising use of breast brachytherapy came as the technology achieved several important milestones. In 2002, the U.S. Food and Drug Administration (FDA) cleared the first balloon-based brachytherapy device (MammoSite, Hologic, Bedford, MA). In April 2004, Medicare authorized reimbursement for balloon brachytherapy treatment. But these developments do not imply clinical efficacy of the treatment, observed lead author Grace Li Smith, MD, PhD, a postdoctoral fellow in the department of radiation oncology, and colleagues.
 |
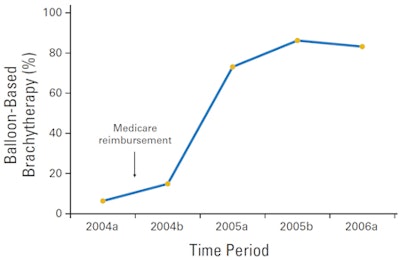
| Percentage of patients treated with brachytherapy (multicatheter or balloon-based) as the sole modality of radiation therapy after breast-conserving therapy (p < 0.001). The "a" after year refers to January through June, and "b" refers to July through December. Images courtesy of ASCO. |
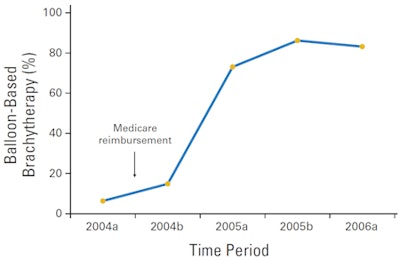 |
| Percentage of patients treated with balloon-based brachytherapy as the sole modality of radiation therapy after breast-conserving therapy (p < 0.001). |
The researchers discovered that where a patient lived and sought treatment most strongly influenced whether she was offered and elected to have breast brachytherapy treatment. The utilization rate was roughly double for women living in western and southern states, compared to those in the northeast. Women who were enrolled in health maintenance organizations for their supplemental insurance also were less likely to have had the treatment.
Other factors promoting breast brachytherapy included living in an area with a higher median income, and living in an area with a lower density of radiation oncologists. Brachytherapy treatment also was more likely in areas with a high density of breast surgeons, which may have reflected better patient access to new technology, the authors speculated.
"The associations with socioeconomic variables, which require further validation, could suggest a unique phenomenon of a reverse disparity, in which more advantaged, better insured patients would be more likely to receive a new, although nonstandard, treatment," they wrote.
The researchers surmised that a lack of clinical evidence demonstrating treatment efficacy was unlikely to have significantly deterred these early adopter older women. Nonclinical factors, including policy, out-of-pocket costs to patients/payors, financial reimbursements to providers, convenience, and socioeconomic issues, seemed to have played important roles. They recommended that further studies be performed, especially with younger patients.
By Cynthia E. Keen
AuntMinnie.com staff writer
January 31, 2011
Related Reading
TARGIT-A data show intraoperative RT equals whole-breast radiation, June 29, 2010
Hologic reports 5-year MammoSite results, November 2, 2010
Long-term APBI outcomes are comparable in low-risk patients, May 31, 2010
New studies hint that APBI could be used in more patients, December 22, 2009
ASTRO publishes APBI guidelines for early-stage breast cancer, July 16, 2009
Copyright © 2011 AuntMinnie.com