A form of intraoperative radiation therapy (IORT) for breast cancer is making headlines this week following news reports of adverse events in patients treated with the technique. The reports indicate that tungsten particles were left behind in patients following the procedure, and at least one lawsuit has been filed.
The reports involve an electronic brachytherapy technique developed by Xoft, a California firm that was acquired in December 2010 by computer-aided detection firm iCAD. On March 21, the New York Times reported that particles from flexible radiation shields used in the procedure left tungsten residue in the breasts of 11 women.
Exactly how many women are affected is as yet unknown. Also unknown are the clinical consequences and whether the story will impact the adoption of intraoperative breast brachytherapy as a treatment for localized breast cancer. The treatment has held promise as being cheaper and more convenient for women than other techniques.
Particles left behind
The New York Times reported that mammograms of women who had undergone lumpectomies and IORT at Hoag Memorial Hospital Presbyterian in Newport Beach, CA, showed hundreds of tiny particles of tungsten in their breast tissue and chest muscles.
The article, by science news reporter Denise Grady, implied that the U.S. Food and Drug Administration (FDA) may have given 510(k) clearance too perfunctorily in June 2009, but the story predominantly focused on issues relating to a patient who had filed a multimillion dollar lawsuit against the hospital and Xoft.
Electronic brachytherapy is a relatively new technology that delivers isotope-free radiation therapy treatments for breast, gynecologic, and skin cancers. It offers the possibility of performing radiation therapy without the radiation shielding requirements used in conventional external-beam treatments.
Intraoperative radiation therapy is typically delivered in the surgical suite immediately following a lumpectomy, providing a rapid, efficient single treatment solution while the patient is anesthetized. Proponents say it limits the amount of radiation dose exposure to healthy organs and tissue, and it's less expensive than accelerated partial-breast irradiation (APBI) procedures or conventional radiotherapy treatments that require 10 to more than 30 treatments.
Intraoperative use of electronic breast brachytherapy technology offers tremendous potential for hospitals to offer a more convenient treatment at a lower cost to a larger number of breast cancer patients. Early positive results from the first of several international and North American clinical trials were published in 2010.
Recipients of the treatment tend to be older than 50 and have a diagnosis of low-risk, early-stage breast cancer with no nodal involvement. Patients who have a confirmed pathological diagnosis of nodal involvement undergo additional radiation therapy.
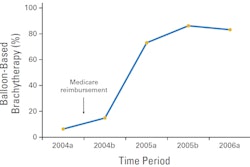
So while it is too soon to compare the long-term effectiveness of this treatment with APBI and conventional external-beam radiation therapy, it shows great promise and is being watched closely by many radiation oncologists. In the U.S., use of IORT breast brachytherapy is expected to proliferate once reimbursement fees are established. In the third quarter of 2010, the American Society for Radiation Oncology (ASTRO) applied for a Category I CPT procedure code for single-fraction IORT, and a reimbursement decision is expected by the end of 2011.
The Axxent eBx system from Xoft and the Intrabeam device from Carl Zeiss Meditec dominate the fledgling IORT electronic breast brachytherapy market, with at least 50 Axxent eBx systems installed worldwide. Based on the potential of the electronic brachytherapy market, iCAD purchased Xoft for the equivalent of $12.9 million in December 2010.
Axxent eBx Systems
At issue in this week's story is Axxent FlexiShield Mini, a flexible radiation shield used in conjunction with Axxent eBx. The shield is a flexible pad that is 12.7 cm in diameter and fabricated from a composite made from tungsten and silicone rubber. The shield is intended to shape the beam from a low-energy radiation therapy source up to 50 kVp, so that surfaces could be shielded.
A number of IORT-specific clinical trials are under way with breast cancer patients using either the Xoft or the Zeiss products. Hoag Memorial Hospital Presbyterian decided to initiate a prospective, single-institution study when it formally launched its IORT breast cancer program in 2010. The protocol for Hoag's clinical trial mandated the use of the Axxent FlexiShield.
Twenty-seven women enrolled, agreeing to a three-year follow-up that included mammograms and breast MRI exams performed at the hospital. In January 2011, the first enrolled patient had her first follow-up mammogram six months after treatment. This revealed hundreds of tiny particles that resembled calcium deposits in the patient's breast and chest muscles. A biopsy was performed and confirmed that the particles were tungsten.
Hoag Memorial filed adverse event reports with the FDA on January 6 and 7, 2011, regarding four patients, stating that it anticipated that "subsequent imaging on 23 additional patients will have similar results on imaging." The adverse reports attributed the "innumerable punctate metallic densities" to "debris left from the tungsten shield placed at the time of intraoperative radiation therapy."
Hoag Memorial issued a statement from its chief quality officer, Dr. Jack Cox, advising that the hospital has stopped scheduling patients for IORT and has notified all patients who had the procedure performed. It would not comment further.
When iCAD was informed of the adverse events, the company immediately recalled the product, contacted the FDA, and notified its customers, meeting with some of them, according to Ken Ferry, president and CEO of iCAD. He told AuntMinnie.com that to date, no other hospital that used the radiation shield has reported similar issues with its patients.
How widespread was use of the company's shield? Ferry did not specify, but explained that shield use was "a mixed bag." Shield use was not required for the electronic breast brachytherapy system for IORT, and a portion of customers, particularly those in Europe, did not use it at all.
Other customers used stainless steel or lead shields, or another manufacturer's flexible shield. Physicians at Little Company of Mary Hospital in Evergreen Park, IL, who presented the first outcomes of breast cancer patients who underwent the procedure using Xoft equipment, confirmed that they have consistently used lead shielding.
"North American customers who used other types of radiation shields told us that the recall did not affect their IORT breast programs, which continue without interruption," Ferry said. "We have not had any reaction from our European customers."
Ferry said that iCAD is going to develop two families of radiation shields -- one that is more rigid, and one that is a flexible composite made of a metal alloy and silicone -- and that the firm is currently in discussions with the FDA.
"We believe that certain types of more rigid products would provide more rapid regulatory approval as a class II product, and enable us to get a shielding product back faster into the market," he explained.
What's next
It's unknown at this time whether the tungsten particles will cause health problems. Karen Riley, a spokesperson for the FDA, said that the agency's toxicologists had found no evidence that the tungsten was toxic or that the patients were harmed. She also acknowledged that the agency had just started its investigation, according to the NY Times article. Very little information exists about the effects of pure tungsten particles embedded in the body.
Members of the Armed Forces Institute of Pathology's division of biophysical toxicology expressed concern about long-term tungsten-related health risks in a literature review published in Military Medicine (2007, Vol. 172:9, pp. 1002-1005). While noting that tungsten and tungsten compounds are considered toxicologically to be relatively safe, lead author Gijsbert B. van der Voet and colleagues noted that "their internalization as embedded shrapnel may be considered a new route for long-term exposure."
"There is not enough information yet to determine whether inhalation, oral, or dermal exposure to tungsten or tungsten compounds can cause cancer in humans," the authors wrote. "However, experimental animal and in vitro studies do not exclude the possibility."
But what is irrefutable is that interpretation of any type of breast imaging examination of these cancer survivors will be a challenge because many of the microparticles resemble calcifications, according to a statement made to Grady by one of the affected women.
A woman who filed a $14.5 million lawsuit in California's Orange County Superior Court in February 2011 against Xoft and Hoag Memorial Hospital Presbyterian said that both her oncologist and the toxicologist she consulted recommended that she have the affected areas surgically removed, the NY Times reported. In addition to a mastectomy, the woman said that she was told that chest muscles would also have to be surgically excised.
In an 8-K form filed with the U.S. Securities and Exchange Commission on March 2, 2011, iCAD stated that it did not anticipate a material impact on its revenues resulting from the recall of the radiation shield. The company also said that it was evaluating possible indemnification claims against Xoft, as well as insurance coverage.