Medical imaging software developer MIM Software has received clearance from the U.S. Food and Drug Administration (FDA) for its MIM Symphony brachytherapy treatment planning system for permanent seed implants.
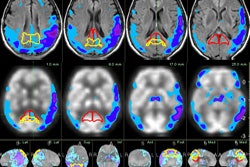
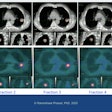
MIM Symphony enables radiation oncologists working with brachytherapy to integrate MRI, CT, ultrasound, and even molecular imaging into treatment planning, according to MIM.
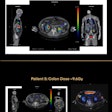
The module features MIM's ReSlicer tool, which allows images to be quickly and easily reoriented for accurate planning and dosimetry. The package can also sum a brachytherapy plan with any other DICOM dose file, giving an accurate representation of the full dose delivered to the patient, MIM said.