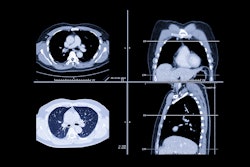
A phase II trial showed that the combination of cetuximab and chemoradiotherapy is well tolerated by patients with inoperable stage III non-small cell lung cancer (NSCLC) and can result in longer median and overall survival, according to a paper published in the June 10 issue of the Journal of Clinical Oncology.
The survival rate in the research from MD Anderson Cancer Center at the University of Texas surpasses that of any previous lung cancer clinical trial performed by the American College of Radiology's (ACR) Radiation Therapy Oncology Group (RTOG).
Between March 2004 and June 2005, 93 participants from 42 RTOG centers were enrolled in the study, with data from 87 eligible patients.
The majority of patients were able to complete the intended therapy, said lead study author and medical oncologist Dr. George Blumenschein Jr. Radiation therapy was delivered according to protocol to 86% of the patients treated, and the cetuximab-chemotherapy combinations were given to a majority of patients.
The median survival time was 22.7 months, and the two-year survival rate was 49.3%.
The results corroborate several large, randomized clinical trials that have validated the efficacy of cetuximab in combination with either chemotherapy or radiation therapy in both NSCLC and squamous cell carcinoma of the head and neck, respectively.
A phase III trial will explore the potential benefits of adding cetuximab to chemoradiotherapy in a similar patient population. So far, more than 90% of the planned 500 study participants have been enrolled.