Medical isotope developer IsoRay Medical has received final approval from the state of Washington's Department of Health to manufacture its GliaSite radiation therapy system balloon catheter device for treating brain cancer.
With previously announced clearance from the U.S. Food and Drug Administration (FDA), Washington state approval is IsoRay's final regulatory hurdle to commence the sale of its GliaSite brain cancer treatment in the U.S.
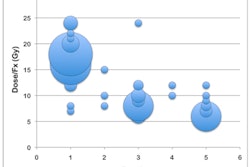
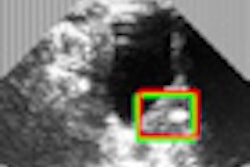
The GliaSite radiation system places a specified high dose of a liquid radiation source in areas most likely to contain cancer after brain tumor removal, the firm said. IsoRay has exclusive worldwide distribution rights for the GliaSite radiation therapy system.