For qualified patients diagnosed with early-stage breast cancer, accelerated partial-breast irradiation (APBI) brachytherapy offers an abbreviated course of treatment. But APBI delivered by a robotic intensity-modulated radiotherapy (IMRT) system offers the additional advantage of being noninvasive.
Unfortunately, only a handful of cancer treatment centers offers this robotic treatment. Why? Reimbursement by private health insurance companies is problematic, as the treatment is considered experimental.
Dr. Sandra Vermeulen, executive director of the Radiosurgery Center at the Swedish Medical Center in Seattle, thinks this is a big mistake. After purchasing a CyberKnife (Accuray) system in 2006, her center was the first in the U.S. to offer this type of breast cancer radiation therapy.
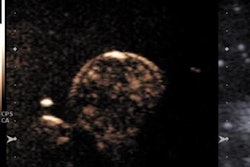
CyberKnife is a frameless, robotic stereotactic radiosurgery system that can deliver targeted, noninvasive external-beam radiation that mimics the dosimetry of breast brachytherapy, but without the implant. It can provide image guidance for continuous tracking of target motion with respiration and patient movement with 1- to 2-mm accuracy; this enables patients to receive a higher dose to a much more constrained space, without the complications that can result from conventional brachytherapy-based APBI.
For breast cancer treatment, using a CyberKnife system can reduce normal tissue inclusion in a planning target volume by approximately half, compared to a treatment plan for 3D conformal radiotherapy and IMRT, Vermeulen said in an interview with AuntMinnie.com.
Because radiation oncologists at the Radiosurgery Center adhere to APBI guidelines released by the American Society for Radiation Oncology (ASTRO) when selecting patients, and because very few qualified patients offered the procedure could get insurance reimbursement, the center has performed only about 20 procedures in five years.
Staff at the Radiosurgery Center sought funding and institutional review board (IRB) approval to conduct a prospective, observational phase II study. Its objective was to evaluate acute and chronic toxicity, cosmesis, and efficacy of APBI using a robotic stereotactic radiosurgery system. However, they were unable to obtain research funds and had to abandon the project.
Vermeulen and colleagues still wanted to share their experiences, though, so they prepared a retrospective analysis for publication of the outcomes of patients treated between June 2009 and May 2011. They continue to offer qualified patients the treatment using the protocol they developed for the proposed study.
The retrospective study
The study, published in Frontiers in Oncology, reported on the outcomes of nine patients with 12 early breast cancers. All were older than 45 years of age and had been diagnosed with stage 0, I, or IIA histologically confirmed invasive nonlobular carcinoma or ductal carcinoma in situ (DCIS). Lesion size was 3 cm or smaller.
All patients had a negative preoperative bilateral breast MRI exam to evaluate disease extent and to rule out additional disease foci in other parts of the ipsilateral breast and the contralateral breast. None of the patients were pregnant, had tumors that involved the skin, had suspicious microcalcifications on mammography, had collagen vascular disease, or had histologically confirmed positive axillary lymph nodes (Front Oncol, November 21, 2011, Vol. 1:43, pp. 1-8).
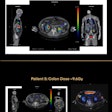
All patients had four or five 2-mm gold fiducial markers sutured into their breast cavity wall at the time they had lumpectomies. The fiducial markers identified the superior, interior, medial, lateral, and deep margins of the cavities.
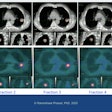
A treatment plan was generally performed three days and up to one week before a five- or 10-fraction course of treatment, which occurred four to five weeks following a patient's breast conservation surgery. Patients received either a 30-Gy radiation dose in five fractions, or 34 Gy in 10 fractions delivered to the target volume.
Patient outcomes
Outcomes have been excellent for all patients, both the ones in the study and those who underwent treatment after May 2011. As might be expected with such a small group of low-risk patients, no breast cancer recurrence has occurred. But compared to the effects of conventional radiation therapy, acute toxicities were minimal.
"Half of the patients we've treated experienced minimal to moderate fatigue, typically for a two- to three-week period following treatment," Vermeulen said. "However, these patients reported that they were experiencing the same level of fatigue after their breast conservation surgery, and during the time interval before they had the radiotherapy treatments."
All patients had increased breast edema following surgery, and this became slightly worse after radiotherapy. About half of the patients showed clinical evidence of breast edema two to four weeks later, but this resolved over two months.
"None of the patients have had skin, muscle, or lung toxicities," Vermeulen said. "Both acute and late cosmetic outcomes have been excellent."
She pointed out that there is no risk of infection or suture leakage, unlike with an APBI breast brachytherapy procedure. Patients also do not have to deal with the discomfort that a balloon inside a lumpectomy cavity may cause.
"The aftereffects of the CyberKnife treatment seem much more tolerable," Vermeulen said. "One of our most recent patients had radiation therapy treatment for a prior breast cancer. She told us that her experience with the CyberKnife treatment was very different, because she didn't experience the debilitating fatigue and horrible radiation burns on her skin that she'd had with the first treatment."
Vermeulen emphasized that CyberKnife treatment, like APBI brachytherapy procedures, is not appropriate for all women. But having performed both, she prefers the robotic IMRT APBI. There is no implanted catheter, which eliminates the need for an additional surgical procedure and reduces the risk of infection, bleeding, and pain. There are fewer technical limitations related to minimum skin spacing and conformation of a balloon catheter to the lumpectomy bed. Finally, considerably less normal tissue is exposed to high radiation dose when compared to 3D conformal APBI.
Clinical trial initiative
Although Vermeulen and colleagues did not succeed in their efforts to start a clinical trial at Swedish Medical Center, radiation oncologist and professor of radiology Dr. Robert Timmerman did. In October 2010, the University of Texas Southwestern Medical Center began enrolling patients in NCT0116220, a phase I study of CyberKnife partial-breast irradiation for early-stage breast cancer.
The objective of the study is to mimic or improve the excellent local control rates seen in the treatment of early-stage breast cancer, while attempting to increase convenience, decrease toxicity, and improve cosmesis compared to other methods of radiation treatment. Its primary outcome measures are to identify the maximum tolerated dose to the lumpectomy cavity, and secondary outcomes are to evaluate dose-limiting toxicity and breast cosmesis three years following treatment.
Enrollment in the trial has now expanded to include patients receiving treatment at Fox Chase Cancer Center and at Stanford Cancer Center, Timmerman said. Patients have been pleased with the use of the technology to limit dose to normal tissues, as well as the convenience of the short-course treatment, he added. The trial is still open for enrollment.
Vermeulen is hopeful that Timmerman's clinical trial will establish the foundation for greater use and encourage private insurance companies to reimburse for this type of breast cancer treatment.
"A lot of women could benefit from this fast, convenient, effective treatment that has few side effects to impact their daily lives, and [that] halves the amount of radiation dose to normal tissues," she concluded.