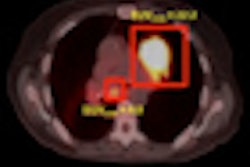
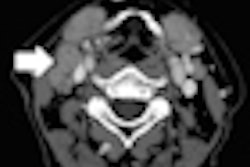
Radiation therapy firm Varian Medical Systems has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for a surface beacon transponder for its Varian Calypso system as a real-time tracking device to monitor motion during radiotherapy for indications anywhere in the body.
The transponder is placed temporarily on the skin for real-time tracking of respiratory and other patient motion during radiotherapy, thereby expanding the number of cancer sites for which the Calypso technology can be used.
Varian garnered the transponder through its acquisition of electromagnetic localization technology developer Calypso Medical Technologies in October 2011.