Treatment couches used to support a patient during intensity-modulated radiotherapy (IMRT) and arc therapy can unknowingly lead to an unacceptable reduction in dose to target structures. Rail placement can also affect the dose delivered.
Radiotherapy treatment planners need to take these factors into consideration, according to a new study from MD Anderson Cancer Center at the University of Texas. The assumption that a treatment table is radiotranslucent is no longer strictly valid (Physics in Medicine and Biology, December 7, 2011, Vol. 56:23, pp. 7435-7447).
Stephen Kry, PhD, an assistant professor of radiation physics, described the problem. "Historically, posterior treatment fields were not common, and when patients were treated with posterior fields they were treated through minimally attenuating couch materials. However, it is now common to treat using a large number of angles around the patient that may intersect different parts of the couch, including the highly attenuating rails. Some recent treatment couch tops are also thicker and therefore more attenuating than historical couches."
Kry also pointed out that data regarding the clinical impact of couch and rail-based attenuation are lacking. To address this issue, the MD Anderson team examined the impact of the Varian Exact Couch (Varian Medical Systems) on IMRT and RapidArc treatments, using Varian's Eclipse v8.6 treatment planning system to model the couch components.
Dose discrepancy
The team planned IMRT (eight-field) and RapidArc (two-arc) treatments for five prostate cancer patients, initially ignoring treatment couch attenuation (no-couch plans). Dose distributions were then recalculated to include the couch and support rails in three configurations: couch top with rails out; couch top with rails in; and couch top only, in which the rails are moved to avoid the beam during IMRT delivery.
Comparing the calculated doses (no-couch plans) with measured doses for the various configurations revealed disagreement of up to 6.2%. On average, all RapidArc plans and rails-out IMRT plans failed or nearly failed the clinical IMRT quality assurance agreement criterion of ± 3%.
The researchers then used dose-volume histograms to evaluate changes in target dose and coverage. For IMRT, the average radiation dose loss from a prescribed 76 Gy was 4.2% (3.2 Gy) for the rails-out plan, with a maximum loss of 5.1% seen in one patient. With the rails in, approximately 2% (1.5 Gy) was lost. For RapidArc plans, dose losses were 3.2% (2.4 Gy) and 2.9% (2.2 Gy) for the rails-out and rails-in plans, respectively, with a maximum loss of 4%.
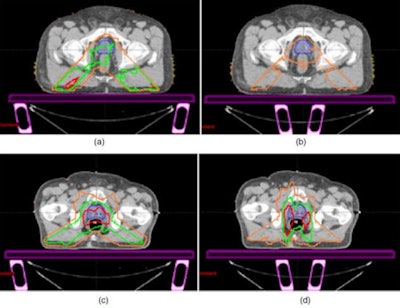 |
| Representative IMRT and RapidArc dose differences between the no-couch scenario and other plan iterations: (a) IMRT rails-out, (b) IMRT rails-in, (c) RapidArc rails-out, and (d) RapidArc rails-in, showing spatially the areas of dose loss due to the couch and rails. Differences of 1 Gy (orange), 2 Gy (green), and 3 Gy (red) are shown. The prostate/CTV is shown in solid colorwash. |
Kry and colleagues then examined the percentage of the target covered by the prescribed dose. When the couch and rails were included, target coverage dropped to 35% (rails-out) and 84% (rails-in). For RapidArc plans, coverage of the prostate dropped from 99% (no-couch) to 18% (rails-out) and 17% (rails-in).
The long-term impact of these dose losses was explored using tumor control probability modeling, revealing an average loss in tumor control of 6.3%. For one IMRT patient, the presence of the couch and rails reduced the tumor control probability by 10.5% to a minimum of 80%.
Less tumor control probability reduction was seen in the RapidArc plans, with inclusion of the couch and rails resulting in a minimum tumor control probability of 78%, 8% less than that predicted by the no-couch scenario for that patient. The authors note, however, that for both delivery modalities, this reduction in tumor control necessitates substantial clinical management of the couch and rails.
The way ahead
The researchers concluded that the treatment couch can cause clinically unacceptable loss of dose and target coverage. One possible solution could be to use a less attenuating couch insert, such as the unipanel mesh couch top available with the Varian Exact couch. A follow-up study revealed that the unipanel mesh does not cause a clinically relevant loss of dose or coverage for IMRT, but still causes an unacceptable loss for RapidArc plans.
Another option would be to use the Eclipse system to account for the couch during planning. While this is a viable approach, extra care is required in positioning the patient with respect to the couch top and rails, as incorrect alignment could introduce even larger dose errors. Furthermore, other planning systems do not offer the ability to add the couch during treatment planning. Manual addition has been attempted using CT images of the couch top; however, it is not feasible to generate CT images of the rails.
"Eclipse could solve the issue for IMRT or RapidArc. But including the couch model for other planning systems, to our knowledge, is something that would be very difficult to do," Kry told medicalphysicsweb. "Hopefully this paper could push the planning system manufacturers to include couch models in future versions of their software."
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.