Accelerated partial-breast irradiation (APBI) brachytherapy appears to be an effective treatment for patients diagnosed with ductal carcinoma in situ (DCIS), according to a study presented at the American Society of Breast Surgeons annual meeting held in Phoenix last week.
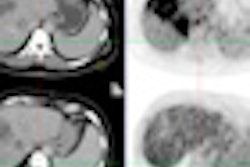
Researchers studied outcomes of 265 women who were followed for a median of 20 months after having breast conservation surgery followed by APBI breast brachytherapy. They found recurrence rates of less than 2% in the study population, which is comparable to outcomes of patients who have conventional whole-breast irradiation, according to lead author Dr. John Einck, a radiation oncologist at the University of California, San Diego (UCSD) Moores Cancer Center.
The data presented are noteworthy because the study is the largest one published to date on the use of breast brachytherapy to treat DCIS. Patients with DCIS and stage 0 breast cancer who are clinically appropriate candidates to receive the five-day treatment are labeled "cautionary" by the American Society for Radiation Oncology (ASTRO) consensus guidelines simply because there are not enough published data, said co-author Dr. Robert Kuske, a radiation oncologist at Arizona Breast Cancer Specialists. This study could help change that, he added.
More than half of the patients were older than 60 years of age, and 30% were between 50 and 60 at the time of treatment. Eighty-eight percent were estrogen-receptor positive, and about half were also prescribed hormone therapy.
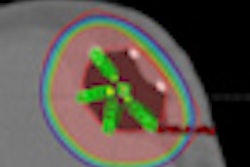
The breast brachytherapy treatments were performed between 2007 and 2010 at 12 sites. The patients were treated with a strut-adjusted volume implant (SAVI) catheter to a total dose of 3,400 cGy delivered in 10 fractions, with two fractions per day for five days.
Late toxicities were experienced by 9% of the total patient cohort, which included 37 women with narrow skin spacing of 5 mm or less between the SAVI applicator and the skin surface. Three patients experienced grade 3 breast pain, two patients experienced grade 3 fat necrosis, and one patient had grade 3 seromas. Eighteen other patients experienced grade 1 and 2 side effects. None of the patients in the study experienced hyperpigmentation.
"Based on these results and other findings, there is emerging data that APBI provides excellent rates of cancer control and low toxicity in properly selected DCIS patients," Einck said.