Radiation oncology products developer Augmenix announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its TraceIT tissue marker.
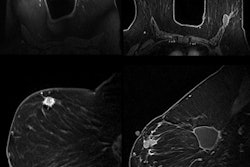
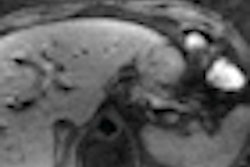
TraceIT is an absorbable tissue marker visible under CT, conebeam CT, MRI, and ultrasound imaging modalities, according to the vendor. It is used to radiographically mark soft tissue prior to surgery and may help simplify image fusion, allowing for improved soft-tissue alignment, Augmenix said.
TraceIT is a synthetic hydrogel consisting primarily of water and iodinated cross-linked polyethylene glycol that is injected through a fine needle. Injections are clearly visible for up to 90 days in the lung, breast, prostate, and other tissues, Augmenix said.