Cancer treatment firm Sirtex Medical is highlighting the results of a multicenter clinical study presented last week at the American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium in San Francisco.
The study, which tracked outcomes of more than 500 patients with metastatic colorectal cancer, reaffirmed the safety and efficacy of selective internal radiation therapy treatment, or radioembolization, using its SIR-Spheres microspheres, according to the vendor.
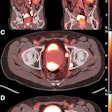
Lead investigator Dr. Andrew Kennedy, director of radiation oncology research at Sarah Cannon Research Institute, presented the retrospective analysis of 548 patients who had been treated with SIR-Sphere microspheres at 11 U.S. cancer treatment centers between July 2002 and November 2011.
All patients had received chemotherapy, and more than 30% had also undergone liver surgery or ablation. Although the median survival of three different groups of patients evaluated did not exceed 13 months, the study authors concluded that the radioembolization appeared to have a favorable risk-benefit ratio in patients with metastatic colorectal cancer who failed chemotherapy, Sirtex said.
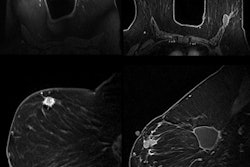
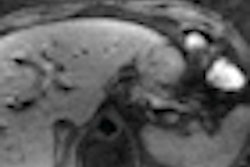
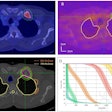
Additionally, results were presented from an independent review of diagnostic imaging exams of 195 patients treated with SIR-Spheres who had measurable lesions at baseline. At the three-month follow-up, 7.6% of the patients showed a partial response and 47.3% experienced stable disease.
The research team noted that response to the radioembolization therapy at three months must be interpreted with caution due to the underestimation of partial response/stable disease or the overestimation of progressive disease. However, even with these caveats, early hepatic radiological response to the therapy appears to predict longer-term prognosis, according to the company.