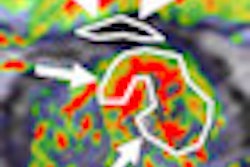
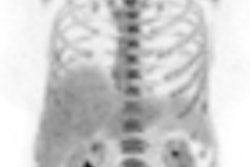
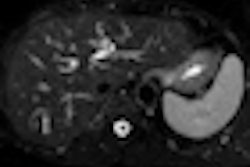
The use of intensity-modulated radiation therapy (IMRT) for gynecologic cancers is increasing, but outcomes data are still lacking. An article published in the March issue of Gynecologic Oncology about the outcomes of high-risk endometrial cancer patients adds to the literature and brings positive news.
Researchers at Memorial Sloan-Kettering Cancer Center reported on 46 patients who underwent IMRT after surgery between November 2004 and November 2009 for stage I to III endometrial cancer. Thirty-six patients were diagnosed with stage III cancer, four had stage I cancer, and six had stage II cancer. More than half (54%) of the patients had positive pelvic lymph nodes (Gynecol Oncol, March 2013, Vol. 128:3, pp. 535-539).
The patients received a median radiation dose of 50.4 Gy, given in fractions of 1.8 Gy. One patient received a dose of 18 Gy and another received 34.2 Gy. Thirty patients also underwent adjuvant chemotherapy, according to lead author Dr. Karin Shih, from the department of surgery, and colleagues.
Three-fourths of the women experienced some degree of toxicity, the most common being diarrhea and proctitis. Two patients experienced nonhematological grade 3 toxicity. Patients who received both chemotherapy and IMRT had more toxicities; this group included all five patients who had grade 3 leukopenia, five of the six patients who had grade 2 leukopenia, and all of the grade 2 anemia and thrombocytopenia patients.
The patients were followed for a median of 52 months, according to the authors. Even though 78% of the women had stage III disease, five-year disease-free survival was 88% and overall survival was 97%. Four patients had cancer recurrence: One developed lung and liver metastases, one had vaginal and lung metastases, and two experienced isolated para-aortic recurrences.
While acknowledging that this study represents a small patient group from a single cancer center, Shih and colleagues said the study will support data emerging from the phase II Radiation Therapy Oncology Group (RTOG) 0418 study. RTOG 0418, which began enrolling patients in 2006, is testing the feasibility of IMRT for patients with either endometrial or cervical carcinoma.