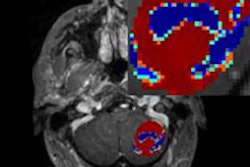
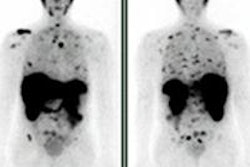
Philips Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Pinnacle3 proton treatment planning module.
The proton module is fully integrated into the Pinnacle³ planning system for radiation therapy, and it enables users to plan either photon- or proton-based radiation therapy, Philips said.