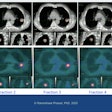
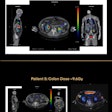
Healthcare technology firm BTG has received U.S. Food and Drug Administration (FDA) 510(k) clearance for LC Bead Lumi, a radiopaque embolic bead designed for embolization of hypervascular tumors and ateriovenous malformations (AVMs).
The next generation of BTG's LC Bead embolic bead technology, LC Bead Lumi is designed to provide interventional radiologists with real-time, visible confirmation of bead location during embolization procedures, according to the vendor.