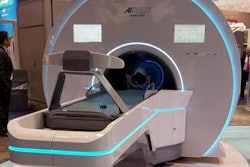
Radiation therapy developer Akesis has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Akesis Galaxy RTx radiosurgery system.
The Akesis Galaxy RTx is a new gamma stereotactic radiosurgery system that's based on Akesis Galaxy.
The RTx model incorporates 30 gamma sources into a rotational design. The configuration gives users more options to shape dose distribution versus traditional, fixed-sector radiation delivery, according to Akesis.