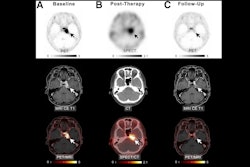
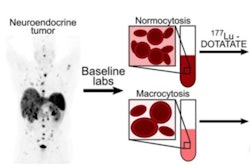
Researchers have identified a risk factor that may be involved in potentially fatal blood disorders in patients undergoing peptide receptor radionuclide therapy (PRRT), according to a study published July 8 in JCO Precision Oncology.
A team of Mayo Clinic physicians evaluated the incidence of clonal hematopoiesis (CH) and cytopenia in patients with neuroendocrine tumors before receiving lutetium-177 (Lu-177) DOTATATE and prospectively followed them over time. They found CH present in 35% and that these patients had lower platelet counts at baseline and remained thrombocytopenic longer on average.
“It is imperative that patients who are at risk of t-MN [therapy-related myeloid neoplasms] are identified early and counseled on this risk,” wrote lead author Yael Kusne, MD, PhD, and colleagues.
Thrombocytopenia – low blood platelets – is a relatively common adverse event of PRRT and often limits patients from a full course of therapy. It can lead to persistent cytopenia and may progress in some cases to t-MN, a potentially fatal stem cell disorder, the authors noted.
To date, it is unclear what mechanisms drive such progression, and in this study, the researchers explored whether PRRT-induced thrombocytopenia may be related to underlying CH in patients with neuroendocrine tumors (NETs).
The group enrolled 37 patients with NETs and treatment plans to receive four cycles of Lu-177 DOTATATE. The median age of the patients was 68 and 51.4% were male. Previous treatment exposures included alkylating agents in 30%, platinum agents in 8%, and external radiation in 13%.
Using a genetic testing technique that identifies mutations related to CH, the researchers found that the prevalence of CH in patients was 35% before PRRT, with age-related CH genetic variants being the most common (DNMT3A, TET2).
In addition, patients with CH had lower baseline platelet counts before PRRT and spent more time in a thrombocytopenic state during PRRT. Post-PRRT, they found that variants in DNA damage response genes were also common.
“Our results indicate that CH may indeed be a risk for hematologic dysfunction during PRRT,” the researchers wrote.
Ultimately, as the indications for PRRT are expanding, including the use of Lu-177 radionuclide therapy in prostate cancer patients, it is important to investigate factors that may predict cytopenia during and after PRRT, the authors noted. They suggested that screening patients for CH before PRRT and monitoring them during and after treatment should be considered.
“Future studies with long-term follow-up will delineate whether CH might be a predictor for higher risk of t-MN after PRRT,” the researchers concluded.
The full study is available here.