A radiopharmaceutical therapy that lengthens progression-free survival for patients with neuroendocrine cancer could help treat meningioma sufferers, according to research presented at the American Society for Radiation Oncology (ASTRO) meeting.
The study findings are hopeful for patients with the condition, presenter Kenneth Merrell, MD, of the Mayo Clinic Alix School of Medicine in Rochester, MN, said in an ASTRO statement.
"Nearly 80% of patients in our study were progression-free after six months," he said. "This rate greatly surpassed the benchmark from prior research, suggesting that radiopharmaceuticals are a promising therapeutic for these patients."
Meningiomas develop in the connective tissue surrounding the brain and spinal cord, and although they tend not to spread to other parts of the body, their growth can lead to "disabling and deadly compression of the nerves and brain," the investigators explained. Standard treatment for these brain tumors is either surgery or external beam radiation therapy, but for patients with what is called refractory meningioma -- a form of the disease that manifests as tumors that continue to grow despite these interventions -- further treatment can be tricky.
Merrell and colleagues explored whether patients with refractory meningioma could benefit from theranostics, an approach that "combines therapy with diagnostics for personalized internal delivery of radiation treatment" and employs radiopharmaceuticals to do so. These drugs are currently used to treat thyroid cancer and some metastatic cancer; Merrell's team investigated whether Lu-177 dotatate in particular, which is used for treating neuroendocrine tumors, would work with meningiomas, since the two cancers are biologically similar.
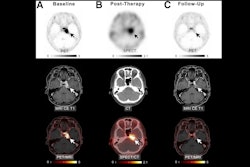
The group conducted a clinical trial that included 20 patients with refractory meningioma categorized as World Health Organization grade II/ III who had grown at a rate of 15% or more over a six-month period. All patients received four infusions of Lu-177 dotatate delivered eight weeks apart. Before each infusion, all underwent a brain MRI exam. The primary endpoint for the study was progression-free survival at six months (exceeding the Response Assessment in Neuro-Oncology [RANO] benchmark of 26% for progression-free survival at six months indicates that a therapy shows promise). The researchers also tracked overall survival and rates of adverse events.
They found the following:
- Progression-free survival was 78% at six months -- exceeding the 26% RANO benchmark -- and one-year overall survival was 88.5%.
- Eastern Cooperative Oncology Group (ECOG) scores at six-month follow-up were 0 (patient fully active) in 55% of study participants; 1 (patient restricted in strenuous activity but otherwise ambulatory) in 35%; and 2 (patient capable of only limited self-care) in 10%.
- Krenning scores (a grading system for neuroendocrine tumors that measures the intensity of tumor uptake on somatostatin receptor imaging) at six-month follow-up were 2 in 45% of patients (uptake equal to or less than the liver); 3 in 45% (uptake greater than the liver); and 4 in 10% (uptake greater than the spleen).
- There were no grade 4 or 5 adverse events (life-threatening consequences requiring urgent intervention or death) connected to the use of Lu-177 dotatate with meningioma patients.
"It appears that Lu-177 dotatate is a safe and rational therapeutic choice with broad eligibility for patients with aggressively growing meningiomas, particularly as alternative therapy options are limited," Merrell concluded.