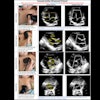
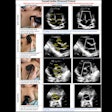
Drug developer Alliance Pharmaceutical of San Diego reports that the Food and Drug Administration has accepted the company's new drug application (NDA) for Imagent, a cardiac ultrasound contrast agent.
Alliance hopes to market Imagent as an agent for improving endocardial border delineation. The company says the phase III clinical trials conducted for Imagent's NDA indicate that the product provides a statistically significant improvement in the visualization of heart walls when compared with standard ultrasound imaging.
Imagent is being developed jointly by Alliance and German pharmaceutical firm Schering, which has exclusive worldwide marketing rights to the product.
By AuntMinnie.com staff writersDecember 15, 1999