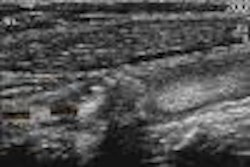
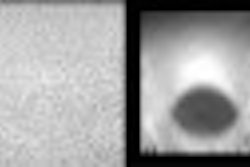
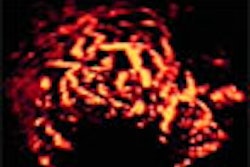
Biomedicom has received 510(k) clearance from the Food and Drug Administration for its SonoReal distance, area, and volume measurement tools for routine clinical obstetrics and gynecology.
SonoReal is the Jerusalem, Israel-based medical imaging software vendor’s 3-D add-on ultrasound imaging platform, which was cleared in June 2000. The company received clearance in March 2000 for its BabyFace unit, a 3-D surface-rendering accessory for ultrasound systems that transforms the flat, frontal view of a fetus into a 3-D image.
By AuntMinnie.com staff writers
April 15, 2002
Related Reading
Biomedicom launches SonoReal in Europe, November 15, 2001
Misonix to deliver SonoReal, April 6, 2001
Misonix gets clearance for 3-D ultrasound unit, March 23, 2000
Copyright © 2002 AuntMinnie.com