CANCÚN, MEXICO - Transrectal ultrasound (TRUS) has proven quite effective for identifying suspicious areas of the prostate at risk of malignancy. Those who perform the procedure will tell you that TRUS has become too popular, in fact, due to referrals of large numbers of patients with high PSA (prostate-specific antigen) screening results.
Widespread screening is surely a welcome development for a disease that kills 300,000 men a year in the U.S. alone, but the problem is that TRUS can't rule out cancer. Prostate cancers have widely varying appearance on TRUS, and such cancers are frequently multifocal. Moreover, many abnormalities identified on TRUS aren't cancer. Other cancers are isogenic, and thus are able to evade ultrasound detection.
Together, these limitations mean that ruling out cancer with any certainty still requires prostate biopsy, an uncomfortable, expensive, and invasive procedure. Whether it is a multiple, sextant biopsy or the more limited targeted kind aimed at suspicious areas, the procedure may be so unpleasant as to discourage regular prostate cancer screening. And so the search continues for a better solution.
In a presentation Thursday at the International Congress of Radiology, Dr. David Cosgrove from the imaging sciences department of Imperial College and Hammersmith Hospital, London, discussed his group's efforts, as well as those of his colleagues in the field, to improve TRUS' ability to detect cancer, and perhaps more important, distinguish aggressive malignancies from low-grade tumors.
 |
| Dr. David Cosgrove. |
"Just as important as the high death rate is the fact that many prostate cancers are slow-growing, and show no tendency to metastasize or invade other structures," Cosgrove said. So the goal in recent years has been to find a method of determining which prostate cancers are most likely to spread.
Color Doppler is an obvious possibility. It has been shown to demonstrate the chaotic and criss-crossed vascularity associated with some tumors since at least 1997, when a study showed that color Doppler-positive regions of the prostate were more likely to have tumor at histology than Doppler-negative regions, Cosgrove said.
In the prostate, however, neovascularity doesn't necessarily equal cancer. A 1998 study showed that PSA scores were closely related to neovascularity on histology, but that neovascularity was not necessarily malignant.
In biopsies of malignant tissue in that study, no significant differences in microvasculature parameters were noted between high and normal color-Doppler flow. One encouraging result, however, was the high correlation of color flow to tumor grade. Biopsies with high color-Doppler flow had average Gleason scores of 6.7, compared with only 5.9 for biopsies with normal flow on color Doppler (P < 0.025). Thus, the cancers that succeeded in getting picked up by color Doppler were more aggressive (Cancer, July 1, 1998, Vol. 83:1, pp. 135-140).
"Interestingly, in the benign lesions, the BPH (benign prostate hypertrophy), the score did relate very closely to the neovascularity on histology," Cosgrove said of the study. "But in 190 carcinomas there wasn't very good correlation at all. Rather disappointing, and it makes the hypothesis of neovascularity (and its association with cancer) less attractive."
On the other hand, a study last year by Kuligowska, Barish, et al found that color Doppler-depicted hypervascularity did correlate with biologically aggressive tumors, but the study also concluded that neither gray-scale nor color Doppler TRUS could replace sextant biopsy for the detection of prostate cancer (Radiology, September 2001, Vol. 220:3, pp. 757-764).
"There are mixed results here," Cosgrove said. "Some say that vascularity can predict aggressivity, some say not.... So we're in a position where I think it's not clear whether color Doppler can help us pick up the cancers that are aggressive."
Another promising technique could clear up the picture, namely microbubble enhancement of color Doppler. But so far, Cosgrove's group has found less than stellar results in three recent studies conducted at Hammersmith Hospital, all of which Cosgrove reported on in his talk.
The first study looked a small group of patients with various types of biopsy-proven prostate cancers. The cohort included 7 patients who had been treated with androgen deprivation therapy, and 8 newly diagnosed cases.
All of patients underwent TRUS following intravenous administration of Levovist ultrasound contrast agent (Schering, Berlin). The data were reviewed on videotape, and interpreted subjectively by consensus of two readers.
The results were scored based on the amount of enhancement; abnormal morphology, including new vessels discovered post-contrast; tortuosity of the neovascular structures; and the number of shunts between vessels. Timing was evaluated based on peak enhancement and time required to return to baseline enhancement.
According to the results, the administration of Levovist revealed neovascularity that was not visible without the agent, and the degree of enhancement was significantly higher in the untreated cancers than in the androgen-deprived group. In addition, a greater number of abnormal features were noted following the administration of Levovist, Cosgrove said.
"Our conclusion from this small series was that Levovist can reveal vascularity, and it certainly showed more neovascular features in active tumor," Cosgrove said, emphasizing that the study was a small series that relied on subjective evaluation of results.
A second study looked at 22 patients in three groups: benign; those with (androgen-deprivation) treated cancers; and newly diagnosed, untreated patients with biopsy-confirmed prostate cancer.
The group used power color Doppler and a quantitative measurement system to evaluate the enhancement response with another microbubble ultrasound contrast agent, SonoVue (Bracco, Milan, Italy). Two dose levels were used -- 2.4 ml and 0.6 ml -- and the group measured the results both in degree of enhancement per milliliter of contrast, and in duration of enhancement.
The results showed that the degree of enhancement was highly dose-dependent -- a sign that speaks well for the agent's stability and perhaps the validity of the results, Cosgrove said. In addition, there was a longer duration of enhancement in untreated cancers in comparison with the benign group (p<0.05). The mean enhancement duration was 27 seconds for the benign subjects, 39 seconds for those with treated cancer, and 49 seconds for the newly diagnosed group with untreated cancers, Cosgrove said.
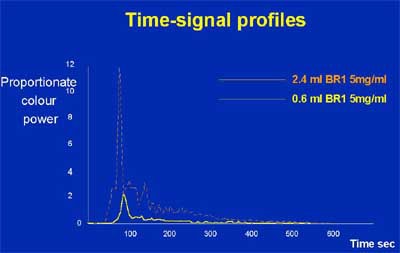 |
| Enhancement increases as SonoVue dose rises. |
"There's a very clear difference (in enhancement) between active cancers and benign lesions," Cosgrove said. "And I find that very interesting. That tells me that there seems to be a truly different interaction of the dynamics of these agents through the lesion."
A final study looked at 24 patients in an effort to determine whether Levovist can predict the response to androgen-deprivation therapy for prostate cancer.
The study used dynamic analysis of contrast response to measure the effect of therapy on enhancement. The intrepid subjects underwent TRUS continually for three months, once at baseline and then again at one and three days after beginning antiandrogen therapy, and again after one, three, and 12 weeks of therapy.
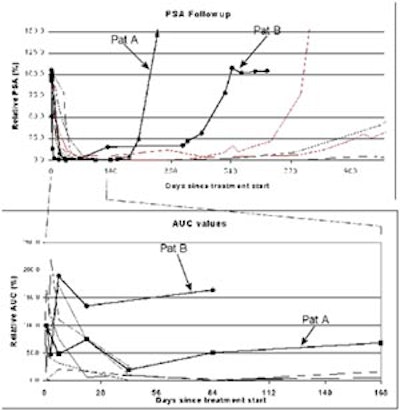
According to the results, the patients fell into two distinct and homogenous groups: those who responded to therapy according to measured enhancement on TRUS, and those who did not respond.
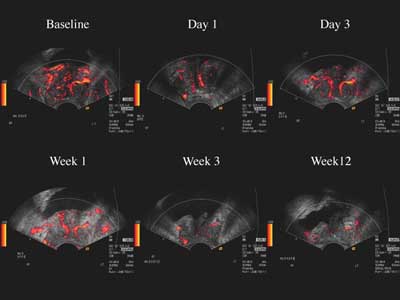 |
| Images of a typical responder to antiandrogen therapy show decreased color Doppler enhancement with Levovist over time. |
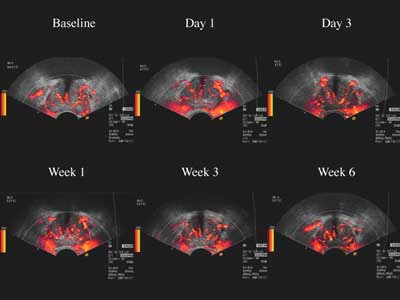 |
| Images of a typical nonresponder to antiandrogen therapy shows steady color Doppler enhancement with Levovist uptake over time. |
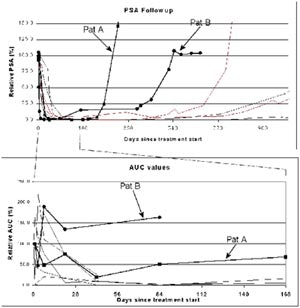 |
| Enhancement compared to PSA for reponders and nonresponders to therapy. Images and graphics courtesy of Dr. David Cosgrove. |
Cosgrove concluded that a demonstration of treatment response to antiandrogen therapy was possible, although nonresponders should have a means of escaping the study earlier to pursue other treatment options before three months have passed. Overall, the prediction of treatment response by contrast-enhanced TRUS is a promising and important avenue for future research, he said.
As for the microbubble contrast agents in general, Cosgrove said they are moderately effective, but not ideally suited to enhancement of prostate cancer imaging with TRUS. He said that color Doppler ultrasound probably adds specificity to the diagnosis of cancer with TRUS, but the increased specificity has not quite been proven. The main problem with the agents, he told AuntMinnie.com after his ICR talk, is that the microbubbles are too big for optimal enhancement.
"The bubbles need to be smaller, ideally," Cosgrove said. "Or some clever way to excite them needs to be developed, which is really the more realistic line of approach. These (manufacturers) don't have millions of dollars to develop a new bubble, test it, and get it through the FDA. They're not going to do that unless they see a big application (as in cardiology or general radiology) -- and this is small stuff."
The likely outcome is that increased enhancement in TRUS will come from a technical advancement that does a better job of exciting the existing microbubble agents, he said. "They are making progress on making the effect happen that we see marvelously in the liver."
By Eric BarnesAuntMinnie.com staff writer
July 5, 2002
Copyright © 2002 AuntMinnie.com