The ultrasound market experienced a relatively quiet year in 2002. The pace of industry consolidation has slowed considerably since blockbuster acquisitions in 2000 by Siemens Medical Solutions and Philips Medical Systems, with the only question remaining being which of the two companies will emerge at the top of the ultrasound heap.
Turf issues were hot this year -- the American Medical Association sparked controversy by announcing its support for universal ultrasound reimbursement, regardless of whether the exams were performed by an imaging professional. The move was hailed by nonimaging specialists eager for a freer hand to use ultrasound, while imaging professionals raised questions about adequate training and oversight.
The question is crucial, as advances in scanner miniaturization are helping ultrasound diffuse into specialties beyond imaging and clinical research is demonstrating that ultrasound can be invaluable as a first-line assessment tool in applications such as trauma treatment. This will be a major trend at this year’s meeting, with a number of companies demonstrating new developments in handheld scanners.
Ergonomic principles continue to set the pace in new system design. Look for new systems that highlight more comfortable scanning positions, in an effort to reduce the high rate of workplace injuries being experienced by sonographers.
Biosound Esaote and Pie Medical USA
Booth #2945 (Bracco Diagnostics)
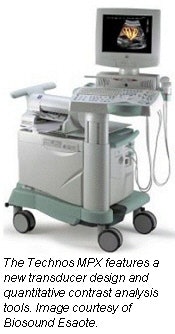
Biosound Esaote will be debuting its all-digital Technos MPX multispecialty ultrasound system in McCormick Place this year. Technos MPX provides image and application enhancement upgrades to the company’s Technos platform.
Technos MPX features quantitative contrast analysis tools, and has the capability of performing online and offline measurement and analysis on either the system itself or on a user’s PC. The system also includes a new transducer design, with the Indianapolis-based company’s tissue enhancement imaging (TEI) harmonic imaging capabilities.
The system uses the Microsoft Windows platform, is DICOM print and store compliant, has a large S-VGA color monitor for reduced eye strain, and a height-adjustable keyboard with right/left swivel for multiple scanning environments. 
Pie Medical USA, which shares facilities with Biosound Esaote in Indianapolis, will be showcasing its Picus 3.0 scanner. Picus is a fully digital and portable system designed for women’s healthcare professionals. It features integrated color flow, B-mode, M-mode, angio, and autotrace spectral Doppler. A new transrectal/transvaginal transducer option is also available for the system.
The product allows three probes to be connected simultaneously, and has a selection of ob/gyn calculation packages available, including amniotic fluid index, twins-quadruplets, fetal-age tables, and fetal weight formulas and ratios. The upgrade also offers improved overall contrast resolution with every transducer, and lateral resolution on all linear transducers, according to the firm.
GE Medical Systems
Booth #4129
GE Medical Systems of Waukesha, WI, will arrive in Chicago with its full stable of ultrasound product offerings to display to attendees. Leading the charge will be the latest models in the company’s Logiq line of scanners.
The Logiq 5 and Logiq Book scanners received Food and Drug Administration 510(k) marketing clearance in January this year. Logiq 5 features the same scanning architecture as the manufacturer’s Logiq 7 and 9 flagship systems, as well as a touch-screen display and standard CRT display. The product is targeted for obstetrics, vascular, small parts, abdominal, and cardiac applications.
Logiq Book is a hand-carried system with full DICOM capabilities such as storage, printing, removable media, and worklist functions. It features an integrated 10.3-inch, high-resolution color monitor, a full-size keyboard, and an image archive that can store more than 4,000 images and cine loops. In addition, it has full cardiac, ob/gyn, vascular, gynecological, renal, urological, and real-time auto Doppler calculation packages with integrated reporting worksheets. A 3-D volume-rendering software package is also available as an option.
GE will also be demonstrating the latest upgrades to its Logiq 7 and 9 series, as well as its Voluson 730 Pro and Expert line of 3-D ultrasound systems.
Medison
Booth #8313
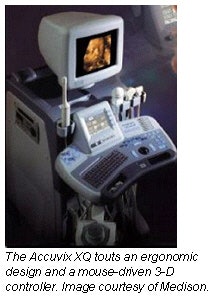
Korean ultrasound firm Medison is bringing two new products to Chicago this December in an effort to gain market share in the U.S. and the rest of the world. The company is debuting a high-end ultrasound system, the Accuvix XQ, and a portable color ultrasound system, the SonoAce Pico.
The internal core of Accuvix XQ will feature 1024-channel digital beamforming technology and frequency compound imaging, as well as the capability to smoothly render an image of a moving fetus and organs in real time. The implementation of harmonic imaging and auto-imaging optimization, as well as a new image-rendering algorithm, have improved both 2-D and 3-D image quality for both color and gray-scale images, according to the firm.
The product has three options for storing images: a high-capacity hard drive, a magneto-optical drive (MOD), and a CD/CD-RW drive. It also incorporates a color LCD touch-screen control panel with a high-resolution layout for increased workflow; four-array active probe connectors, and an open port for pencil-type probe connection.
Accuvix XQ boasts a mouse-driven 3-D controller that allows a user to manipulate scanned images in real time. SonoView II software comes installed for comprehensive image management that permits thumbnail image display, direct printing and reporting, and image e-mail via DICOM network functionality. The system is also able to export images in JPEG, TIFF, and BMP file formats. The software also includes a quantifiable data analysis package, with technologies such as multiplanar image display, segmentation of the rendered images, and automatic volume measurement.
Accuvix XQ is due to be officially introduced in spring of 2003, and is expecting FDA 510(k) approval later this quarter, according to Seoul-based Medison.
Building on the success of its SonoAce line of portable ultrasound systems, the firm also will be demonstrating its SonoAce Pico color digital system in its RSNA booth. Targeted at ob/gyn, internal medicine, cardiology, and radiology users, the product has a form factor the size of a business briefcase.
The unit features 64-channel digital beamforming and harmonic imaging, as well as pulsed-wave spectral, power, and color Doppler. It also includes freehand 3-D capabilities for 2-D and 3-D images in both color and black and white.
The product’s operating platform is based on Linux and can be connected with a local area network, so that system maintenance and upgrades can be accomplished remotely. The system has SonoView Lite image-management software pre-installed for image filing and management. A USB port enables the device to connect to an external MOD or a CD/RW drive for increased storage capacity in addition to its high-capacity hard drive.
SonoAce Pico is slated for market introduction in the first quarter of 2003, and Medison is expecting FDA 510(k) clearance for the product later this quarter, according to firm.
Go to page:
Biosound to Medison
Philips to Toshiba



















