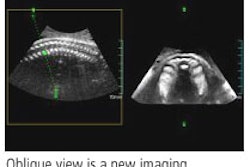
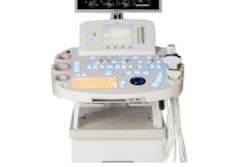
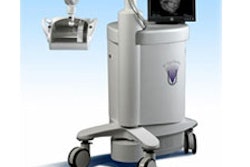
Radiation therapy firm North American Scientific (NAS) has received regulatory approval from the People's Republic of China State Food and Drug Administration (SFDA) to sell its BAT SXi, an ultrasound-based localization system used to view cancerous tumors prior to the delivery of radiation therapy.
The image-guided radiation therapy (IGRT) system is designed to be compatible with all linear accelerators and most treatment-planning systems, NAS said. The SFDA approval clears the way for NAS to sell BAT SXi in China, according to the Chatsworth, CA-based firm.
By AuntMinnie.com staff writers
February 21, 2006
Related Reading
NAS gets FDA clearance for brachytherapy needle kit, February 7, 2006
NAS fiscal 2005 results impacted by Nomos purchase, January 10, 2006
NAS readies serial tomotherapy package, October 10, 2005
NAS gets China OK for Peacock, September 21, 2005
NAS trims losses in Q3, September 6, 2005
Copyright © 2006 AuntMinnie.com