Medical device manufacturer St. Jude Medical of St. Paul, MN, reported that it has received European CE Mark approval for its QuickOpt timing cycle optimization application.
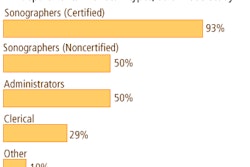
The product has been shown to be comparable to a traditional echocardiography procedure for determining optimal conduction delays in clinical trials, the firm said. St. Jude Medical said the QuickOpt procedures in the study were completed within two minutes, while echocardiography optimization typically takes between 30 and 120 minutes and requires a manual investigation by a sonographer.
The product has been launched on the European market, and the company has filed a premarket approval (PMA) supplement application with the U.S. Food and Drug Administration for programmer software with the QuickOpt feature.
By AuntMinnie.com staff writers
June 16, 2006
Related Reading
GE, St. Jude Medical partner on cardiac imaging, May 16, 2006
St. Jude gets FDA nod for Angio-Seal VIP, March 17, 2006
St. Jude successful with US cardiac ablation, December 21, 2004
Copyright © 2006 AuntMinnie.com