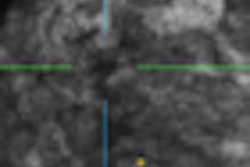
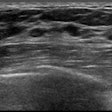
Automated breast ultrasound (ABUS) technology developer U-Systems has become the first vendor to receive U.S. Food and Drug Administration (FDA) clearance to market a device specifically for use in screening women with dense breasts.
Following the recommendation of the FDA's Radiological Devices Panel in May, the agency today approved U-Systems' premarket approval (PMA) application for its somo.v ABUS system to be utilized in screening applications as an adjunct to mammography for asymptomatic women with dense breast tissue.
Somo.v currently has 510(k) clearance for diagnostic use as an adjunct to mammograms, but the new indication will aim to increase breast cancer detection in asymptomatic women (BI-RADS assessment category 1 or 2) with dense breast parenchyma (BI-RADS composition/density 3 or 4) following a negative screening mammogram, according to the company.
"We are extremely excited that we will now be able to offer radiologists and breast imaging experts this important tool," said U-Systems President and CEO Ron Ho. "It's long been our mission to provide a tool for those 40% of women [with dense breasts] who are underserved by mammography alone."
Somo.v has already received the European CE Mark and Canadian Health Ministry approval as an adjunct to mammography for breast cancer screening.
Based on clinical trial data, Ho said the company believes its ABUS technology will be widely adopted for this application.
"The data that the FDA used as the basis for our approval shows that our tool, along with mammography, finds 30% more cancers in women who have normal mammograms," he told AuntMinnie.com. "We think that with the lay knowledge that mammography itself is an imperfect tool, particularly in women with dense breasts, and the fact that women with dense breasts are at a higher risk for having cancer, we think this will really create a demand for the product."
Somo-v ABUS is approved for use in women who have not had previous clinical breast intervention, such as a surgery or biopsy, since this might alter the appearance of breast tissue in an ultrasound image, according to the FDA. As part of the approval, the FDA is requiring that U-Systems provide thorough training for physicians and technologists using the ABUS device, and that the manufacturer provide each facility with a manual clearly defining system tests required for initial, periodic, and yearly quality control measures.
U-Systems plans to use a combination of direct sales and partnerships with other distributors to roll out the expanded application, according to Ho.
"We have a lot of pent-up demand," Ho said. "As soon as we get the approval order from the FDA, we've got plans to commercialize immediately."
Current somo.v users will be able to begin utilizing the system in cancer detection for women with dense breasts.