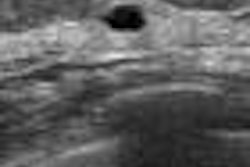
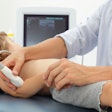
Australian handheld ultrasound developer Signostics has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Signos RT ultrasound system.
The handheld ultrasound device will be marketed under the brand name of Sonimage P3 by distribution partner Konica Minolta Medical Imaging, Signostics said.