Ultrasound computer-aided detection (CAD) technology can improve reader accuracy and diagnostic confidence for distinguishing between benign and malignant breast lesions, according to Japanese researchers.
In an observer study, a team from Mie University found that its internally developed ultrasound CAD system yielded significant gains in accuracy as well as changes in mean diagnostic confidence level.
"The CAD scheme is considered to be useful for clinicians in making differential diagnosis of masses in ultrasound images," said Dr. Yumi Kashikura. She presented the research during a scientific session at RSNA 2012.
Ultrasound could be useful in breast cancer screening because it is able to identify some cases that are difficult to detect in mammography screening, such as small invasive cancers in dense breasts. However, ultrasound has lower specificity than mammography, Kashikura said.
"If clinicians can make a differential diagnosis more accurately on ultrasonographic images, they may be able to achieve more effective screening," she told AuntMinnie.com. "Therefore, we thought if the CAD could improve a clinician's performance, it could be one of the solutions for the problem."
The researchers investigated their CAD scheme, which provides histological classifications of breast masses on ultrasound images. Because each lesion's histological type has a complex combination of image features, the group developed the CAD scheme using an artificial neural network to analyze nine image features: circularity, polygonal shape, lobulated shape, irregular shape, depth-width ratio, indistinctness in margin, heterogeneousness in internal echoes, echo level in internal echoes, and echo level in posterior echoes, Kashikura said.
Still in development, the CAD algorithm can currently analyze lesions with four histological classifications: cyst, fibroadenoma, ductal carcinoma in situ (DCIS), and invasive ductal carcinoma (IDC). Prior research using the CAD system has found sensitivities of 85.3%, 86.9%, 81.4%, and 85.1%, respectively, for the four histological classifications. Overall sensitivity and specificity based on classification results were 95.6% and 90.5%, she said.
In the current study, the researchers wanted to evaluate the usefulness in differential diagnosis of providing readers with CAD-estimated likelihood of histological classifications.
In the trial, seven clinicians read a dataset of ultrasound images; all were selected from exams obtained at their institution in 2010 on an Aplio XG SSA-790A scanner (Toshiba Medical Systems) with a 12-MHz linear-array transducer. For inclusion, the studies had to have a mass lesion size of 4 mm to 25 mm, be pathologically proved, and lack artifacts.
A total of 390 images met these criteria. To select moderately difficult cases, three experts were asked to provide their confidence level regarding mass malignancy or benignity on a continuous rating scale. Based on these results, the researchers selected 100 images for the final observer study. The 100 images included 50 benign masses (25 cysts and 25 fibroadenomas) and 50 malignant masses (25 IDC and 25 DCIS), she said.
After an initial read of the image, the seven observers, which included three expert and four experienced breast imagers, were asked to mark their confidence in malignancy on a continuous rating scale of 0 (definitely benign) to 1 (definitely malignant). The CAD software then displayed the likelihood of histological classification, and the observers were asked again to mark their confidence level.
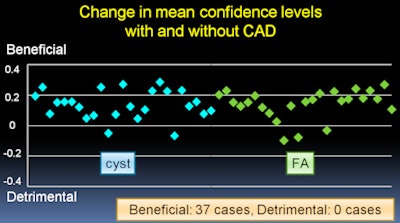
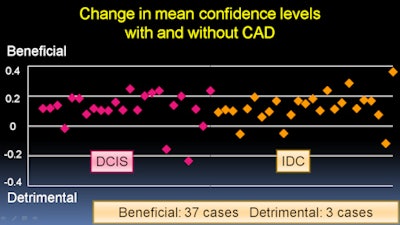
The results were analyzed with multireader, multicase receiver operator characteristics (ROC) analysis. In addition, changes more than 0.1 in mean confidence level after viewing CAD results were considered to be either a benefit or a detriment, Kashikura said.
The average area under the curve (AUC) for all observers increased from 0.714 without CAD to 0.889 with CAD. The difference was statistically significant (p = 0.0001).
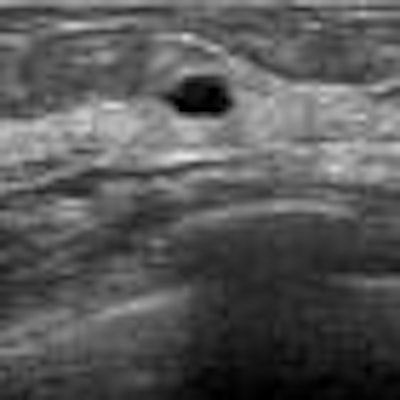
 |
| All images courtesy of Dr. Yumi Kashikura. |
 |
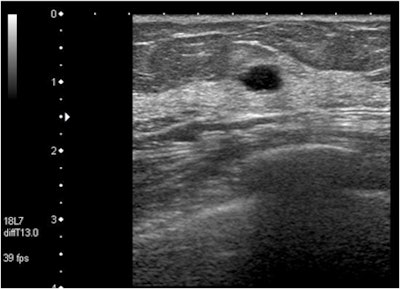 |
| In this case of invasive ductal carcinoma, CAD increased the mean confidence level from 0.503 without CAD to 0.739. |
"Presenting the likelihood of the histological classification as evaluated by the CAD scheme improved the clinicians' performance," she said.
In the next phase of the software's development, the researchers would like to extend its capabilities to include detection of hypoechoic lesions, Kashikura said.