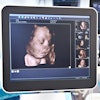
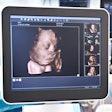
Tomographic ultrasound developer Delphinus Medical Technologies received another 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its SoftVue system.
The additional regulatory clearance is for depicting color imaging based on tissue characteristics from the capture of transmission signals. SoftVue is powered with circular, volumetric transducer technology and has ultrafast 360 electronic sequencing, enabling transducer elements to both send and receive signals.
SoftVue captures reflection echoes from all directions around the breast and gathers transmitted signals coming through the breast, the firm said.