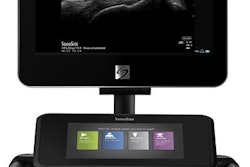
Ultrasound developer Fujifilm SonoSite has received U.S. Food and Drug Administration (FDA) 510(k) clearance and the European CE Mark for its new SII mountable point-of-care ultrasound (POCUS) system.
Targeted at regional anesthesia, vascular access, and trauma applications, SII is portable and can be used across multiple environments, according to the vendor. A zero-footprint option is available for space-constrained rooms, Fuji said.
The system features a new touchscreen interface that adaptively adjusts to the clinical application it's being used for, Fuji SonoSite said. In addition, users can switch between transducers via an embedded dual-transducer connector and by tapping twice on the screen. A new stand also offers elevated transducer holders and additional storage while minimizing the footprint, according to the firm.
Fuji SonoSite is also directing attention to the system's DirectClear technology, which is designed to increase penetration and contrast resolution on select transducers.