EchoNous has received U.S. Food and Drug Administration (FDA) clearance for EchoNous Vein, a handheld ultrasound scanner designed to help nurses with the placement of peripheral intravenous catheters.
EchoNous Vein is intended to address the challenge that nurses face in finding a healthy vein for catheter placement. Studies have shown that placing peripheral IV catheters can be challenging due to chronic illness, chemotherapy, and obesity, as well as drug abuse. The company cited research indicating that first-attempt IV catheter insertion fails in up to 26% of adults and 54% of children.
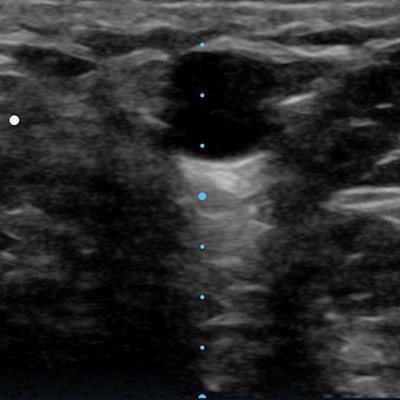
EchoNous Vein addresses the problem with ultrasound-based technology that scans at depths of 1 cm to 5 cm to quickly visualize both superficial and deeper veins. The scanner operates with simple two-button controls, and it includes settings that are optimized for children and adults.
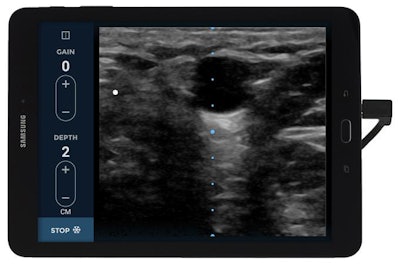 EchoNous Vein is designed to help with the insertion of peripheral IV catheters. Photo courtesy of EchoNous.
EchoNous Vein is designed to help with the insertion of peripheral IV catheters. Photo courtesy of EchoNous.Product testing has shown that the scanner's frequency profile and optimized gain and depth presets are particularly helpful to healthcare personnel for evaluating the veins of pediatric patients, who traditionally are a challenging patient population for IV insertion, according to the company.
In addition to announcing the launch of EchoNous Vein, the company said it plans to merge its Signostics brand and its Uscan bladder scanner product under the single EchoNous brand.



















