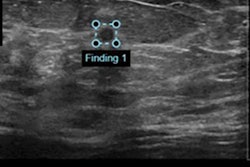
The U.S. Food and Drug Administration (FDA) has cleared artificial intelligence (AI) software developer Koios Medical's machine-learning software for the classification and diagnosis of breast cancer.
Koios DS Breast 2.0 helps clinicians analyze breast ultrasound images and generates a probability of malignancy score with a corresponding BI-RADS category, the company said. It can be used with most PACS platforms and is available on GE Healthcare's Logiq E10, according to Koios.