The U.S. Food and Drug Administration (FDA) has approved Delphinus Medical Technologies' new 3D whole-breast ultrasound system for detecting cancer in women with dense breasts.
The company's SoftVue 3D whole-breast ultrasound tomography system is intended as an assist to digital mammography for screening asymptomatic women with dense tissue.
"[FDA approval] is a huge validation of our hard work and the uniqueness of the system," CEO Mark Forchette told AuntMinnie.com.
 The SoftVue 3D whole-breast ultrasound tomography system. Image courtesy of Delphinus Medical Technologies.
The SoftVue 3D whole-breast ultrasound tomography system. Image courtesy of Delphinus Medical Technologies.Approximately 40% of U.S. women have dense breast tissue, and density is considered a risk factor for breast cancer. A federal mandate was established in 2019 requiring mammography facilities to inform women if they have dense breasts after every mammogram.
While mammography is the standard in breast cancer screening, using it alone for dense breasts can lead to missed cancers. MRI has been touted for its high sensitivity in dense tissue, but its low specificity means it can also detect benign lesions, potentially leading to unnecessary biopsies and surgeries.
"We need additional ways to find these cancers that are hidden in mammograms. If we can find them early, it can be lifesaving," Delphinus board member Dr. Rachel Brem from George Washington University Hospital in Washington, DC, told AuntMinnie.com.
Brem herself is a breast cancer survivor, having received her cancer diagnosis via ultrasound two months after receiving a negative mammogram.
"I feel so fortunate that ultrasound found my breast cancer. I was one of the lucky ones," she said. "We want to increase the pool of the lucky ones."
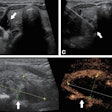
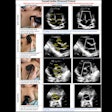
During a SoftVue exam, the woman lies on her stomach with her breasts submerged in a warm-water bath. After the breasts are stabilized and centered with a disposable gel pad, the device scans each breast from chest wall to nipple tip in an average of three minutes. The exam is performed with a 360° ring transducer and captures new images every 2 mm. The technology captures the reflection, speed, and attenuation of sound waves as they pass through tissue, which helps distinguish normal from abnormal lesions, the company said. The signals are analyzed using algorithms that provide cross-sectional slices of the entire volume of breast tissue.
Delphinus gathered data on the device's efficacy in a reader study of 32 radiologists from 10 clinical sites across the U.S. that included images from about 8,500 patients. The system increased sensitivity by 20% and specificity by 8%, and it identified up to 20% more cancers with greater accuracy than mammography alone, according to the firm.
With the FDA's premarket approval, SoftVue exams can be performed at the same appointment as screening mammograms.
"First and foremost, this is a win for patients. But I think for our employees and everyone here, it's a huge thing that we're super excited about," Forchette said. "It takes a culture of people who are willing to swing for the fence and try to do breakthrough things."