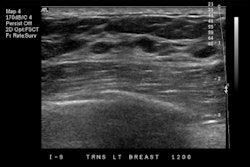
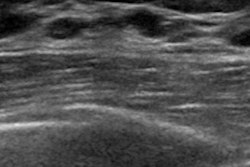
The U.S. Food and Drug Administration (FDA) has cleared Koios Medical's Koios DS, an artificial intelligence (AI)-based software platform used to diagnose thyroid and breast cancer.
The company said Koios DS was developed using ultrasound data from a network of 48 sites around the world, with the aim of aiding physicians in diagnosing diseases and improving speed to treatment while reducing avoidable surgeries.
Koios also said the software aligns the system's generated findings directly to the American College of Radiology's BI-RADS and TI-RADS rating systems, as well as the American Thyroid Association's system for tissue classification, scoring, and patient management.
The software is also compatible with major PACS workstation viewers and is integrated into the Logiq E10 ultrasound scanner from GE Healthcare.