The U.S. Food and Drug Administration (FDA) has cleared point-of-care ultrasound developer Butterfly Network's artificial intelligence (AI)-based handheld ultrasound device for lung health assessment.
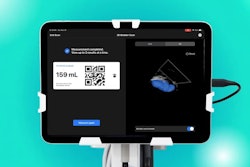
Auto B-line Counter produces a B-line count from a six-second ultrasound clip. B-lines are bright, vertical lines that denote wetness in the lungs and are correlated with pulmonary air-space diseases such as congestive heart failure, chronic obstructive pulmonary disease, pneumonia, and COVID-19, according to Butterfly. It features a percent counting method that assigns numbers to B-lines by the percentage of affected rib space around the lungs and counts separate B-lines, the company said.
Butterfly expects to launch Auto B-line Counter in early summer this year.