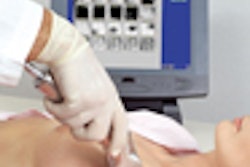
Breast cancer diagnosis developer Biofield has begun a post-market surveillance (PMS) survey in Europe of its Biofield Diagnostic System (BDS), the company said. BDS provides objective, bioelectronic results that are immediately available to physicians. The device is designed to complement physical examination and breast imaging modalities in women with suspicious breast lesions, according to the Atlanta-based firm.
The survey is expected to include 200-300 patients with previously identified breast lesions. Biofield intends to use the anticipated results of the survey to support the clinical utility and marketing of BDS, and intends to release the system in Europe by late 2000. The company anticipates submitting a pre-market approval (PMA) application to the Food and Drug Administration after data analysis in hopes of achieving U.S. clearance.
By AuntMinnie.com staff writersAugust 31, 2000
Copyright © 2000 AuntMinnie.com