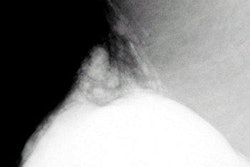
Imaging Diagnostic Systems announced at last week’s RSNA conference that its CT laser mammography system (CTLM) has been recommended for CE marking. The recommendation is subject to review by Underwriters Laboratories Notified Body, which permits distribution in the European markets, according to the company.
In the U.S., IMDS is seeking premarket approval (PMA) from the Food and Drug Administration for use as an adjunct to mammography. IMDS expects to complete the submission process by early 2001, according to the Ft. Lauderdale, FL-based company.
By AuntMinnie.com staff writersDecember 6, 2000
Related Reading
Imaging Diagnostic gets more financing, August 21, 2000
Copyright © 2000 AuntMinnie.com