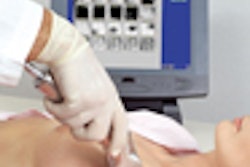
Imaging Diagnostic Systems has submitted to the FDA the second module of its application for pre-market approval of its CT laser mammography (CTLM) system. The company said it anticipates finalizing the module submission process early this year.
The Plantation, FL-based firm has targeted the CTLM system for use as an adjunct to mammography in the detection of breast abnormalities. CTLM is a non-invasive procedure that does not expose the patient to radiation or require breast compression.
By AuntMinnie.com staff writersJanuary 3, 2001
Related Reading
CT laser mammography system nears European approval, December 6, 2000
Imaging Diagnostic gets more financing, August 21, 2000
IDS names medical director, May 18, 2000
IDSI bolsters financial position, February 23, 2000
Copyright © 2001 AuntMinnie.com