Theoretically at least, MRI is considered more suitable for breast imaging than the x-ray systems that now predominate. For unlike x-ray mammography, MRI can distinguish between fatty and glandular breast tissues. It can detect some breast cancers, such as infiltrating lobular carcinomas, more sensitively than standard mammography. If one also considers MRI's superiority in imaging dense breast tissue and differentiating postoperative scarring from recurrent cancer, its growing role in mammography seems, well, natural.
But MRI's advantages haven't yet compensated for its lack of specificity. Benign conditions such as adenosis, fibroadenoma, and fibrocystic changes can be mistaken for cancer on MRI, and the modality can't see the clusters of microcalcifications that often correlate with malignancy. So as MRI technology has improved, researchers have turned to contrast enhancement and other techniques in efforts to expand breast MRI's limited range of applications.
A presentation at the 2001 European Congress of Radiology in Vienna described one such approach: a new image fusion method being developed at the University of Oxford in the U.K. The technique matches dual-view mammograms with contrast-enhanced MRI images to correlate subtle pathological indicators that would be missed on either modality alone.
"Over the past two years we've been working with the possibility of combining image data between mammography and contrast-enhanced MRI with gadolinium," said Christian Behrenbruch, a medical physicist at the Oxford Medical Vision Laboratory, and product development manager for spin-off company OMIA.
"The underlying idea is the sort of functional representation of MRI vs. the very detailed structural representation of mammography," he said. "In the clinical setting we've found three main applications for this sort of data fusion: generally younger patients, or patients who have had tissue regeneration due to hormone replacement therapy; patients who have multifocal disease and impalpable tumors, because the sensitivity of mammography may not be so good in patients with dense breasts; and of course DCIS."
The study assessed the fusion tool's accuracy in 12 patients with a wide range of pathology, including both malignant and benign disease, Behrenbruch said.
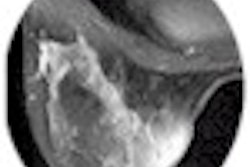
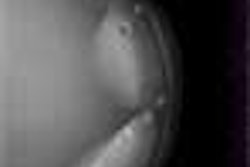
All patients underwent both fast spoiled gradient-echo (FSPGR) T1-weighted MR imaging (TR/TE 8.9/4.2 ms, a=10º) with gadolinium; and standard mammography including mediolateral (ML) and craniocaudal (CC) views.
The images were then fused with the MammoFusion software developed at the university's vision laboratory and at John Radcliffe Hospital in Oxford. MammoFusion uses image-processing algorithms and nonrigid deformable models to coregister the ML and CC mammograms with the 3-D contrast-enhanced MRI volumes, Behrenbruch explained.
"The first step is to project the contrast enhancement from the 3-D volume using a pharmacokinetic model -- an analysis of gadolinium uptake in the MRI images," he said. And we project that contrast enhancement information -- so what we're actually doing is comparing a projection of the contrast characteristics of the breast with the mammographic representation, and we do that in the approximate projection frame."
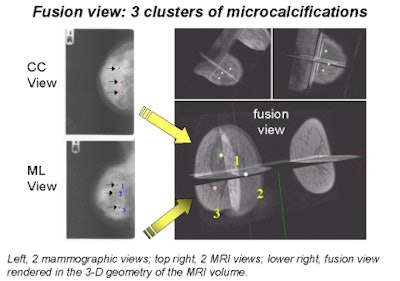 |
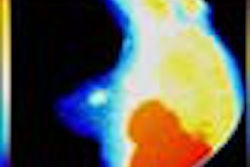
Behrenbruch showed images (above and below) of one study subject, a 26-year-old woman with DCIS. The patient had recurrent malignancy following an upper quadrant mastectomy and reconstruction. Three clusters of microcalcifications were visible in both mammographic views. When core biopsy was unsuccessful, the patient was referred for MR imaging of the breast, Behrenbruch said. The MRI and mammographic images were subsequently fused and reconstructed using MammoFusion. Correlating calcifications seen in the mammogram with the MRI data revealed subtle enhancement consistent with DCIS.
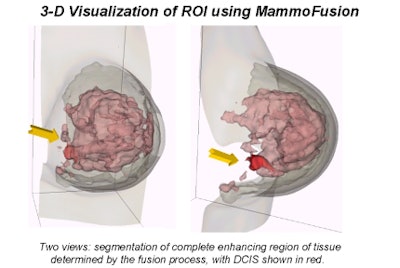 |
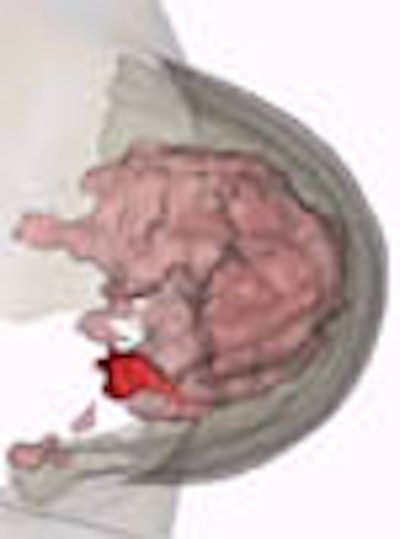
"We use a nonrigid matching algorithm," Behrenbruch said. "Essentially we're trying to warp the mammogram onto the shape of those MRI volume projections. And it's a two-step process, first using the boundary shape, and then using the internal landmarks that can be detected. [The image above] is the more complex deformation using the landmarks."
Based on an evaluation of the centroid and extent of regions of interest (ROI), the image registration error in the 12 patients averaged approximately 3.5 voxels for all cancers, and approximately 5 voxels for large, poorly differentiated carcinomas. That's not bad, he said, considering that difference between the clinicians' and the software's assessment of tumor location is only about 20%. The accuracy could be improved, he noted, but said the technique has already eliminated an important obstacle.
"From what I understand, many MR radiologists don't look at mammograms at all, because they feel that they are unable to make that correspondence in their head just because the geometry of the imaging is so different," he said.
In summary, Behrenbruch said his group's method has a wide range of potential clinical uses.
"The idea is not to replace the radiologist or the radiologist's assessment of the MRI volume, it's designed to enable him to take information from the screening radiologists, which is quite useful," Behrenbruch said. "It's an interactive tool, in the sense that radiologists can intuit the matching process, and they do feel quite comfortable that the fusion is meaningful. We're interested in doing some larger studies at the moment with some biopsy validations to improve the capabilities of the tool."
By Eric BarnesAuntMinnie.com staff writer
April 27, 2001
Note: MammoFusion is being developed and clinically evaluated through a technology partnership between Oxford University Medical Vision Laboratory and spin-off companies OMIA/OXIVA. All images courtesy of Christian Behrenbruch.
Click here to post your comments about this story. Please include the headline of the article in your message.
Copyright © 2001 AuntMinnie.com