Mammography companies are stepping up to answer the call for new technologies that will improve the efficacy of screening mammography. On the RSNA floor, look for new advances in computer-aided detection (CAD), non-x-ray breast imaging techniques, and full-field digital mammography (FFDM).
The advances may help breast imaging advocates address some of the concerns that continue to swirl around screening mammography. On the positive side, 2002 saw the publication of a Swedish study in Cancer in July that claimed that mammography reduces cancer deaths by up to 44%. Proponents of screening touted the article as an effective rebuttal to doubts raised by a Danish paper in The Lancet two years ago that criticized mammography research.
Mammography’s reputation got a black eye in June, however, when the New York Times published a two-part broadside that excoriated the performance of some radiologists who read mammograms. The series claimed that many general radiologists did not read enough films to stay competent in mammography, and that organized radiology was not adequately policing breast imagers.
At the same time, mammography centers continue to struggle to provide screening services. High malpractice insurance costs, low reimbursement levels, and personnel shortages are making some facilities wonder if mammography screening is worth the hassle.
Against this backdrop, CAD and FFDM products are becoming vital assets in the fight to improve screening mammography. Radiologists first looked warily at CAD, but are now welcoming it with open arms as an ally in solving the screening conundrum.
CAD vendors at RSNA will highlight new capabilities with their systems, with improving workflow a common refrain. CAD vendors are also beginning to expand out of breast imaging and into other applications, such as CT screening for lung cancer.
FFDM also shows promise as an integral piece of a hub-and-spoke model of breast screening, in which digital images are acquired at remote facilities and transmitted via teleradiology to be read at centralized departments staffed by breast experts. In 2002, Hologic received FDA approval for its Lorad Selenia FFDM system, joining GE Medical Systems and Fischer Imaging on the U.S. market. On the RSNA floor, look for other mammography vendors to highlight their ongoing work in FFDM.
Also at the show, look for alternative breast imaging products that use ultrasound waves, high-energy light-emitting diodes (LEDs), or infrared light. Such systems could become valuable adjuncts to screening mammography by helping reduce its high false-positive rate.
Advanced Imaging Technologies
Booth #5716
The Preston, WA-based firm will highlight the Avera Breast Imaging System, which uses real-time ultrasound to create multiplanar images with no radiation or compression. Advanced Imaging first introduced Avera at last year’s RSNA meeting.
CADx Systems
Booth #6534
CADx will feature Second Look, its FDA-approved, stand-alone CAD workstation that uses computer algorithms to identify suspicious areas on mammograms. The Beavercreek, OH, company will also highlight its work-in-progress Second Look Digital system for use with FFDM systems.
CADx will demonstrate Second Look Remote, for remote facilities that require CAD, as well as Second Look with CT, for lung cancer detection. CADx is also pursuing the use of its technology with other applications, such as imaging of colon cancer and cardiovascular disease.
Computerized Thermal Imaging
Booth #4148
Lake Oswego, OR-based CTI will showcase BCS 2100, the company’s work-in-progress computerized thermal breast imaging system. The unit is designed to be used as a follow-up to abnormal mammograms, helping physicians distinguish between benign and malignant lesions and therefore reduce the number of benign breast biopsies performed. BCS 2100 uses infrared technology and a heat-sensitive camera to detect and analyze heat patterns in the breast, and requires no compression of breast tissue. The FDA is scheduled to review the unit and make a recommendation regarding clearance in December.
Confirma
Booth #8352
Confirma will launch CADstream, a magnetic resonance image-processing device developed for physicians who interpret breast MR studies. CADstream identifies contrast uptake and washout curves, creates angiogenesis maps and subtraction images, and performs image registration, multiplanar reformatting, and maximum intensity projections (MIPs).
CADstream was cleared by the FDA in January and will be available in January 2003, according to the Kirkland, WA-based firm. In October, the company installed a beta unit at First Hill Diagnostic Imaging in Seattle.
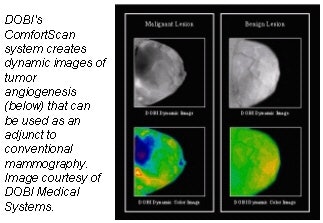
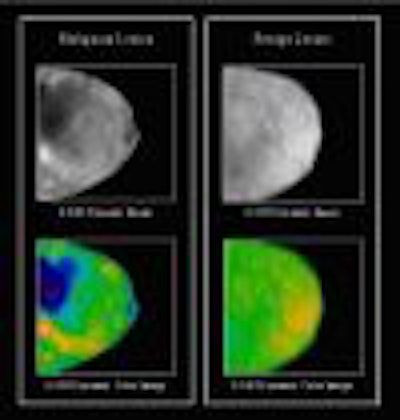
DOBI Medical Systems plans to feature its ComfortScan system at this year’s show. Based
on technology that detects tumor angiogenesis, ComfortScan focuses on dynamic functional imaging rather than a singular morphological image, according to the Mahwah, NJ, company.
The unit uses high-intensity light-emitting diodes (LEDs), a charge-coupled device (CCD) camera, and image-processing algorithms to highlight areas of vascular development common to malignant breast tumors.
DOBI is seeking FDA clearance for ComfortScan as an adjunct to mammography, and hopes to complete its application by late 2003. ComfortScan is already being sold outside the U.S. under the CE Mark, the European equivalent of FDA approval which it received in April 2000.

Booth #4345
Fischer’s SenoScan TrueView 25/50 Digital Mammography unit will be on display in the Denver-based company’s booth. Fischer has upgraded SenoScan’s software with customizable defaults for reading protocols, automatic image processing, and patient set-up parameters.
SenoScan can be used in either standard screening or diagnostic modes, with resolutions of 50 microns and 25 microns, respectively. The unit delivers up to 60% less radiation dose to patients due to Fischer’s slot-scanning technology, and has a field-of-view large enough to accommodate all breast sizes without repositioning, according to the company.
SenoScan also employs an open computer platform that gives users flexibility to incorporate CAD into their workflow and to view and diagnose images remotely, Fischer said.
GE Medical Systems
Booth #4129
GE will present upgrades to its Senographe 2000D full-field digital mammography system, the first FFDM unit to be cleared by the FDA. The upgrades include a mobile configuration of the unit and a CAD system provided by partner R2 Technology. The Waukesha, WI, firm will also feature an upgraded paddle design for its Senographe DMR+ film-screen mammography system.

Booth #3500
Hologic will highlight its Lorad Selenia FFDM system, cleared by the FDA in October, which uses DirectRay amorphous selenium flat-panel detectors. In the DirectRay digital conversion process, photoconductors convert x-rays to electronic signals without first converting them to light. This removes light diffusion and protects image sharpness, according to the company.
Selenia detectors also include Hologic’s High Transmission Cellular (HTC) grid, which reduces radiation scatter. Hologic of Bedford, MA, expects to begin shipping Selenia in the U.S. in 2003.
Next page:
iCAD through Siemens