Imaging Diagnostic Systems (IDSI) of Fort Lauderdale, FL, this week submitted a 510(k) premarket notification to the U.S. Food and Drug Administration (FDA) for its CT laser mammography (CTLM) breast imaging device.
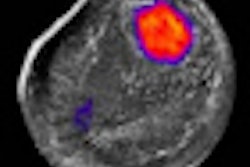
With the device, a patient lies face down on the scanning bed for approximately 10 minutes until the scan is completed. CTLM uses laser technology and proprietary computed algorithms, rather than x-rays and breast compression, to create 3D views of the blood supply in the breast.
The images can be used to complement current breast imaging modalities and provide additional information on the nature of breast abnormalities.
Related Reading
IDSI completes Indian install, July 12, 2010
IDSI gets Australia nod, February 2, 2010
IDSI completes Malaysian install, September 22, 2009
IDSI hires strategic advisor, June 11, 2009
IDSI appoints managing director for India, April 30, 2009
Copyright © 2010 AuntMinnie.com